A compatibility study on the glycosylation of 4,4'-dihydroxyazobenzene
- PMID: 35515580
- PMCID: PMC9053478
- DOI: 10.1039/d0ra02435j
A compatibility study on the glycosylation of 4,4'-dihydroxyazobenzene
Abstract
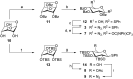
Photoresponsive glycoconjugates based on the azobenzene photoswitch are valuable molecules which can be used as tools for the investigation of carbohydrate-protein interactions or as precursors of shape-switchable molecular architectures, for example. To access such compounds, glycosylation of 4,4'-dihydroxyazobenzene (DHAB) is a critical step, frequently giving heterogeneous results because DHAB is a challenging glycosyl acceptor. Therefore, DHAB glucosylation was studied using nine different glycosyl donors, and reaction conditions were systematically varied in order to find a reliable procedure, especially towards the preparation of azobenzene bis-glucosides. Particular emphasis was put on glucosyl donors which were differentiated at the primary 6-position (N3, OAc) for further functionalisation. The present study allowed us to identify suitable glycosyl donors and reaction conditions matching with DHAB, affording the bis-glycosylated products in fair yields and good stereocontrol.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare about the authors.
Figures


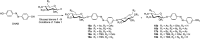
Similar articles
-
Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity.Acc Chem Res. 2018 Mar 20;51(3):628-639. doi: 10.1021/acs.accounts.7b00449. Epub 2018 Feb 22. Acc Chem Res. 2018. PMID: 29469568 Review.
-
Stereoselective synthesis of α-glucosides with glucosyl (Z)-Ynenoates as donors.Carbohydr Res. 2023 Jan;523:108710. doi: 10.1016/j.carres.2022.108710. Epub 2022 Oct 31. Carbohydr Res. 2023. PMID: 36370627
-
Glycosylation from the non-reducing end using a combination of thioglycoside and glycosyl sulfoxide as the glycosyl donor and the acceptor.Chem Pharm Bull (Tokyo). 2010 May;58(5):758-64. doi: 10.1248/cpb.58.758. Chem Pharm Bull (Tokyo). 2010. PMID: 20460812
-
2-nitroglycals as powerful glycosyl donors: application in the synthesis of biologically important molecules.Acc Chem Res. 2008 Aug;41(8):1059-73. doi: 10.1021/ar7002495. Epub 2008 Jul 4. Acc Chem Res. 2008. PMID: 18598060 Review.
-
Application of Interrupted Pummerer Reaction Mediated (IPRm) Glycosylation in Natural Product Synthesis.Chem Rec. 2020 Jul;20(7):743-751. doi: 10.1002/tcr.201900097. Epub 2020 Feb 26. Chem Rec. 2020. PMID: 32103624 Review.
Cited by
-
Azo-Bridged Dextran: A Photoresponsive Sustainable Material with Photo-Tunable Mechanical Properties.Biomacromolecules. 2025 Mar 10;26(3):1737-1747. doi: 10.1021/acs.biomac.4c01508. Epub 2025 Feb 6. Biomacromolecules. 2025. PMID: 39912756 Free PMC article.
-
Sulfur and Azobenzenes, a Profitable Liaison: Straightforward Synthesis of Photoswitchable Thioglycosides with Tunable Properties.Chemistry. 2022 Jul 11;28(39):e202200354. doi: 10.1002/chem.202200354. Epub 2022 Jun 1. Chemistry. 2022. PMID: 35537915 Free PMC article.
-
ZnI2-Mediated cis-Glycosylations of Various Constrained Glycosyl Donors: Recent Advances in cis-Selective Glycosylations.Molecules. 2024 Oct 4;29(19):4710. doi: 10.3390/molecules29194710. Molecules. 2024. PMID: 39407638 Free PMC article. Review.
-
Acid Violet 3: A Base-Activated Water-Soluble Photoswitch.J Phys Chem A. 2024 Feb 1;128(4):785-791. doi: 10.1021/acs.jpca.3c07128. Epub 2024 Jan 18. J Phys Chem A. 2024. PMID: 38236752 Free PMC article.
References
-
- Zollinger H., in Color Chemistry: Syntheses, Properties and Applications of Organic Dyes and Pigments, ed. P. M. Wallimann, Wiley-VCH, Weinheim, 2003, vol. 7, pp. 165–254
-
- Johnson L. Ringstrand B. Kaszynski P. Liq. Cryst. 2009;36:176–185. doi: 10.1080/02678290902759210. - DOI
- Laurent N. Lafont D. Dumoulin F. Boullanger P. Mackenzie G. Kouwer P. H. Goodby J. W. J. Am. Chem. Soc. 2003;125:15499–15506. doi: 10.1021/ja037347x. - DOI - PubMed
- Cook A. G. Wardell J. L. Brooks N. J. Seddon J. M. Martínez-Felipe A. Imrie C. T. Carbohydr. Res. 2012;360:78–83. doi: 10.1016/j.carres.2012.07.018. - DOI - PubMed
- Abraham S. Paul S. Narayan G. Prasad S. K. Rao D. S. Jayaraman N. Das S. Adv. Funct. Mater. 2005;15:1579–1584. doi: 10.1002/adfm.200500089. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

