Aldehyde catalysis - from simple aldehydes to artificial enzymes
- PMID: 35515689
- PMCID: PMC9056934
- DOI: 10.1039/d0ra06651f
Aldehyde catalysis - from simple aldehydes to artificial enzymes
Abstract
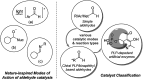
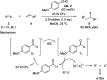
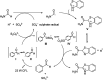
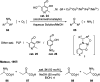
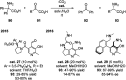
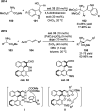
Chemists have been learning and mimicking enzymatic catalysis in various aspects of organic synthesis. One of the major goals is to develop versatile catalysts that inherit the high catalytic efficiency of enzymatic processes, while being effective for a broad scope of substrates. In this field, the study of aldehyde catalysts has achieved significant progress. This review summarizes the application of aldehydes as sustainable and effective catalysts in different reactions. The fields, in which the aldehydes successfully mimic enzymatic systems, include light energy absorption/transfer, intramolecularity introduction through tether formation, metal binding for activation/orientation and substrate activation via aldimine formation. Enantioselective aldehyde catalysis has been achieved with the development of chiral aldehyde catalysts. Direct simplification of aldehyde-dependent enzymes has also been investigated for the synthesis of noncanonical chiral amino acids. Further development in aldehyde catalysis is expected, which might also promote exploration in fields related to prebiotic chemistry, early enzyme evolution, etc.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



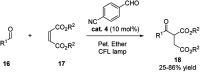



















Similar articles
-
The diarylprolinol silyl ether system: a general organocatalyst.Acc Chem Res. 2012 Feb 21;45(2):248-64. doi: 10.1021/ar200149w. Epub 2011 Aug 17. Acc Chem Res. 2012. PMID: 21848275
-
Organocatalysis using aldehydes: the development and improvement of catalytic hydroaminations, hydrations and hydrolyses.Chem Commun (Camb). 2017 Dec 12;53(99):13192-13204. doi: 10.1039/c7cc07352f. Chem Commun (Camb). 2017. PMID: 29131221
-
Catalytic asymmetric organozinc additions to carbonyl compounds.Chem Rev. 2001 Mar;101(3):757-824. doi: 10.1021/cr000411y. Chem Rev. 2001. PMID: 11712502 Review.
-
Asymmetric Catalysis within the Chiral Confined Space of Metal-Organic Architectures.Small. 2019 Aug;15(32):e1804770. doi: 10.1002/smll.201804770. Epub 2019 Feb 4. Small. 2019. PMID: 30714307 Review.
-
Enantioselective Carbonyl Catalysis Enabled by Chiral Aldehydes.Angew Chem Int Ed Engl. 2019 May 20;58(21):6818-6825. doi: 10.1002/anie.201808700. Epub 2019 Mar 12. Angew Chem Int Ed Engl. 2019. PMID: 30216640 Review.
Cited by
-
Repurposing a Catalytic Cycle for Transient Self-Assembly.J Am Chem Soc. 2024 Aug 21;146(33):23289-23296. doi: 10.1021/jacs.4c05871. Epub 2024 Aug 11. J Am Chem Soc. 2024. PMID: 39127918 Free PMC article.
-
Automated in vivo enzyme engineering accelerates biocatalyst optimization.Nat Commun. 2024 Apr 24;15(1):3447. doi: 10.1038/s41467-024-46574-4. Nat Commun. 2024. PMID: 38658554 Free PMC article. Review.
-
Chiral aldehyde catalysis enables direct asymmetric α-substitution reaction of N-unprotected amino acids with halohydrocarbons.Chem Sci. 2023 May 3;14(21):5665-5671. doi: 10.1039/d3sc01294h. eCollection 2023 May 31. Chem Sci. 2023. PMID: 37265737 Free PMC article.
-
A New Age of Biocatalysis Enabled by Generic Activation Modes.JACS Au. 2024 May 31;4(6):2068-2080. doi: 10.1021/jacsau.4c00247. eCollection 2024 Jun 24. JACS Au. 2024. PMID: 38938808 Free PMC article. Review.
-
Chiral aldehyde-nickel dual catalysis enables asymmetric α-propargylation of amino acids and stereodivergent synthesis of NP25302.Nat Commun. 2022 Nov 26;13(1):7290. doi: 10.1038/s41467-022-35062-2. Nat Commun. 2022. PMID: 36435942 Free PMC article.
References
-
-
Selected reviews:
- Dalko P. I. Moisan L. Angew. Chem., Int. Ed. 2001;40:3726. doi: 10.1002/1521-3773(20011015)40:20<3726::AID-ANIE3726>3.0.CO;2-D. - DOI - PubMed
- Dalko P. I. Moisan L. Angew. Chem., Int. Ed. 2004;43:5138. doi: 10.1002/anie.200400650. - DOI - PubMed
- Seayad J. List B. Org. Biomol. Chem. 2005;3:719. doi: 10.1039/B415217B. - DOI - PubMed
- Pellissier H. Tetrahedron. 2007;63:9267. doi: 10.1016/j.tet.2007.06.024. - DOI
- Dondoni A. Massi A. Angew. Chem., Int. Ed. 2008;47:4638. doi: 10.1002/anie.200704684. - DOI - PubMed
- Gaunt M. J. Johansson C. C. C. McNally A. Vo N. T. Drug Discovery Today. 2007;12:8. doi: 10.1016/j.drudis.2006.11.004. - DOI - PubMed
- Bertelsen S. Jørgensen K. A. Chem. Soc. Rev. 2009;38:2178. doi: 10.1039/B903816G. - DOI - PubMed
- Buckley B. R. Farah M. M. Annu. Rep. Prog. Chem., Sect. B: Org. Chem. 2011;107:102. doi: 10.1039/C1OC90020J. - DOI
- Buckley B. R. Annu. Rep. Prog. Chem., Sect. B: Org. Chem. 2013;109:189. doi: 10.1039/C3OC90015K. - DOI
- Oliveira V. d. G. Cardoso M. F. d. C. Forezi L. d. S. M. Catalysts. 2018;8:605. doi: 10.3390/catal8120605. - DOI
- Sideri I. K. Voutyritsa E. Kokotos C. G. Org. Biomol. Chem. 2018;16:4596. doi: 10.1039/C8OB00725J. - DOI - PubMed
- van der Helm M. P. Klemm B. Eelkema R. Nat. Rev. Chem. 2019;3:491. doi: 10.1038/s41570-019-0116-0. - DOI
- Reyes-Rodríguez G. J. Rezayee N. M. Vidal-Albalat A. Jørgensen K. A. Chem. Rev. 2019;119:4221. doi: 10.1021/acs.chemrev.8b00583. - DOI - PubMed
-
-
-
Recent reviews:
- Pascal R. Eur. J. Org. Chem. 2003;2003:1813. doi: 10.1002/ejoc.200200530. - DOI
- Li B.-J. El-Nachef C. Beauchemin A. M. Chem. Commun. 2017;53:13192. doi: 10.1039/C7CC07352F. - DOI - PubMed
- Li S. Chen X.-Y. Enders D. Chem. 2018;4:2026. doi: 10.1016/j.chempr.2018.08.011. - DOI
- Gong L.-Z. Sci. China: Chem. 2019;62:3. doi: 10.1007/s11426-018-9345-5. - DOI
- Shimoda Y. J. Synth. Org. Chem., Jpn. 2019;77:369. doi: 10.5059/yukigoseikyokaishi.77.369. - DOI
- Wang Q. Gu Q. You S.-L. Angew. Chem., Int. Ed. 2019;58:6818. doi: 10.1002/anie.201808700. - DOI - PubMed
- Chen J. Liu Y. E. Dong X. Shi L. Zhao B. Chin. J. Chem. 2019;37:103.
- Theodoropoulou M. A. Nikitas N. F. Kokotos C. G. Beilstein J. Org. Chem. 2020;16:833. doi: 10.3762/bjoc.16.76. - DOI - PMC - PubMed
-
-
-
Recent examples and reviews:
- Kofoed J. Reymod J.-L. Darbre T. Org. Biomol. Chem. 2005;3:1850. doi: 10.1039/B501512J. - DOI - PubMed
- Koch K. Schweizer W. B. Eschenmoser A. Chem. Biodiversity. 2007;4:541. doi: 10.1002/cbdv.200790049. - DOI - PubMed
- Amedjkouh M. Brandberg M. Chem. Commun. 2008;2008:3043. doi: 10.1039/B804142N. - DOI - PubMed
- Cleaves II H. J. Precambrian Res. 2008;164:111. doi: 10.1016/j.precamres.2008.04.002. - DOI
- Benner S. A. Kim H.-J. Kim M.-J. Ricardo A. Cold Spring Harbor Perspect. Biol. 2010;2:a003467. - PMC - PubMed
- Kajjout M. Hebting Y. Albrecht P. Adam P. Chem. Biodiversity. 2012;9:714. doi: 10.1002/cbdv.201100124. - DOI - PubMed
- Meinert C. Myrgorodska I. de Marcellus P. Buhse T. Nahon L. Hoffmann S. V. d'Hendecourt L. L. S. Meierhenrich U. J. Sicence. 2016;352:208. doi: 10.1126/science.aad8137. - DOI - PubMed
- López-Islas A. Colín-García M. Negrón-Mendoza A. Int. J. Astrobiol. 2019;18:420. doi: 10.1017/S1473550418000368. - DOI
- Eckhardt A. K. Bergantini A. Singh S. K. Schreiner P. R. Kaiser R. I. Angew. Chem., Int. Ed. 2019;58:5663. doi: 10.1002/anie.201901059. - DOI - PubMed
- Bernhardt H. S. Life. 2019;9:19. doi: 10.3390/life9010019. - DOI - PMC - PubMed
-
-
-
For recent reviews:
- Reckenthäler M. Griesbeck A. G. Adv. Synth. Catal. 2013;355:2727. doi: 10.1002/adsc.201300751. - DOI
- Xuan J. Lu L.-Q. Chen J.-R. Xiao W.-J. Eur. J. Org. Chem. 2013;2013:6755. doi: 10.1002/ejoc.201300596. - DOI
- Koike T. Akita M. Top. Organomet. Chem. 2014;48:371. doi: 10.1007/3418_2014_80. - DOI
- Wang C. Lu Z. Org. Chem. Front. 2015;2:179. doi: 10.1039/C4QO00306C. - DOI
- Aukauloo A. and Leibl W., Chapter Three - Bioinspired Photocatalysis, Advances in Botanical Research, ed. B. Robert, Academic Press, 2016, vol. 79, p. 63
- Kokotos C. G. Org. Biomol. Chem. 2018;16:4596. doi: 10.1039/C8OB00725J. - DOI - PubMed
- Zeitler K., Metal-free Photo (redox) Catalysis, Visible Light Photocatalysis in Organic Chemistry, ed. C. R. J. Stephenson, T. P. Yoon and D. W. C. MacMillan, Wiley-VCH, Weinheim, 2018
- Zhou Q.-Q. Zou Y.-Q. Lu L.-Q. Xiao W.-J. Angew. Chem., Int. Ed. 2019;58:1586. doi: 10.1002/anie.201803102. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources

