Aldehyde catalysis - from simple aldehydes to artificial enzymes
- PMID: 35515689
- PMCID: PMC9056934
- DOI: 10.1039/d0ra06651f
Aldehyde catalysis - from simple aldehydes to artificial enzymes
Abstract
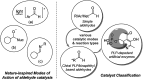
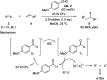
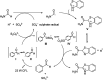
Chemists have been learning and mimicking enzymatic catalysis in various aspects of organic synthesis. One of the major goals is to develop versatile catalysts that inherit the high catalytic efficiency of enzymatic processes, while being effective for a broad scope of substrates. In this field, the study of aldehyde catalysts has achieved significant progress. This review summarizes the application of aldehydes as sustainable and effective catalysts in different reactions. The fields, in which the aldehydes successfully mimic enzymatic systems, include light energy absorption/transfer, intramolecularity introduction through tether formation, metal binding for activation/orientation and substrate activation via aldimine formation. Enantioselective aldehyde catalysis has been achieved with the development of chiral aldehyde catalysts. Direct simplification of aldehyde-dependent enzymes has also been investigated for the synthesis of noncanonical chiral amino acids. Further development in aldehyde catalysis is expected, which might also promote exploration in fields related to prebiotic chemistry, early enzyme evolution, etc.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



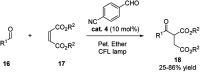



















References
-
-
Selected reviews:
- Dalko P. I. Moisan L. Angew. Chem., Int. Ed. 2001;40:3726. doi: 10.1002/1521-3773(20011015)40:20<3726::AID-ANIE3726>3.0.CO;2-D. - DOI - PubMed
- Dalko P. I. Moisan L. Angew. Chem., Int. Ed. 2004;43:5138. doi: 10.1002/anie.200400650. - DOI - PubMed
- Seayad J. List B. Org. Biomol. Chem. 2005;3:719. doi: 10.1039/B415217B. - DOI - PubMed
- Pellissier H. Tetrahedron. 2007;63:9267. doi: 10.1016/j.tet.2007.06.024. - DOI
- Dondoni A. Massi A. Angew. Chem., Int. Ed. 2008;47:4638. doi: 10.1002/anie.200704684. - DOI - PubMed
- Gaunt M. J. Johansson C. C. C. McNally A. Vo N. T. Drug Discovery Today. 2007;12:8. doi: 10.1016/j.drudis.2006.11.004. - DOI - PubMed
- Bertelsen S. Jørgensen K. A. Chem. Soc. Rev. 2009;38:2178. doi: 10.1039/B903816G. - DOI - PubMed
- Buckley B. R. Farah M. M. Annu. Rep. Prog. Chem., Sect. B: Org. Chem. 2011;107:102. doi: 10.1039/C1OC90020J. - DOI
- Buckley B. R. Annu. Rep. Prog. Chem., Sect. B: Org. Chem. 2013;109:189. doi: 10.1039/C3OC90015K. - DOI
- Oliveira V. d. G. Cardoso M. F. d. C. Forezi L. d. S. M. Catalysts. 2018;8:605. doi: 10.3390/catal8120605. - DOI
- Sideri I. K. Voutyritsa E. Kokotos C. G. Org. Biomol. Chem. 2018;16:4596. doi: 10.1039/C8OB00725J. - DOI - PubMed
- van der Helm M. P. Klemm B. Eelkema R. Nat. Rev. Chem. 2019;3:491. doi: 10.1038/s41570-019-0116-0. - DOI
- Reyes-Rodríguez G. J. Rezayee N. M. Vidal-Albalat A. Jørgensen K. A. Chem. Rev. 2019;119:4221. doi: 10.1021/acs.chemrev.8b00583. - DOI - PubMed
-
-
-
Recent reviews:
- Pascal R. Eur. J. Org. Chem. 2003;2003:1813. doi: 10.1002/ejoc.200200530. - DOI
- Li B.-J. El-Nachef C. Beauchemin A. M. Chem. Commun. 2017;53:13192. doi: 10.1039/C7CC07352F. - DOI - PubMed
- Li S. Chen X.-Y. Enders D. Chem. 2018;4:2026. doi: 10.1016/j.chempr.2018.08.011. - DOI
- Gong L.-Z. Sci. China: Chem. 2019;62:3. doi: 10.1007/s11426-018-9345-5. - DOI
- Shimoda Y. J. Synth. Org. Chem., Jpn. 2019;77:369. doi: 10.5059/yukigoseikyokaishi.77.369. - DOI
- Wang Q. Gu Q. You S.-L. Angew. Chem., Int. Ed. 2019;58:6818. doi: 10.1002/anie.201808700. - DOI - PubMed
- Chen J. Liu Y. E. Dong X. Shi L. Zhao B. Chin. J. Chem. 2019;37:103.
- Theodoropoulou M. A. Nikitas N. F. Kokotos C. G. Beilstein J. Org. Chem. 2020;16:833. doi: 10.3762/bjoc.16.76. - DOI - PMC - PubMed
-
-
-
Recent examples and reviews:
- Kofoed J. Reymod J.-L. Darbre T. Org. Biomol. Chem. 2005;3:1850. doi: 10.1039/B501512J. - DOI - PubMed
- Koch K. Schweizer W. B. Eschenmoser A. Chem. Biodiversity. 2007;4:541. doi: 10.1002/cbdv.200790049. - DOI - PubMed
- Amedjkouh M. Brandberg M. Chem. Commun. 2008;2008:3043. doi: 10.1039/B804142N. - DOI - PubMed
- Cleaves II H. J. Precambrian Res. 2008;164:111. doi: 10.1016/j.precamres.2008.04.002. - DOI
- Benner S. A. Kim H.-J. Kim M.-J. Ricardo A. Cold Spring Harbor Perspect. Biol. 2010;2:a003467. - PMC - PubMed
- Kajjout M. Hebting Y. Albrecht P. Adam P. Chem. Biodiversity. 2012;9:714. doi: 10.1002/cbdv.201100124. - DOI - PubMed
- Meinert C. Myrgorodska I. de Marcellus P. Buhse T. Nahon L. Hoffmann S. V. d'Hendecourt L. L. S. Meierhenrich U. J. Sicence. 2016;352:208. doi: 10.1126/science.aad8137. - DOI - PubMed
- López-Islas A. Colín-García M. Negrón-Mendoza A. Int. J. Astrobiol. 2019;18:420. doi: 10.1017/S1473550418000368. - DOI
- Eckhardt A. K. Bergantini A. Singh S. K. Schreiner P. R. Kaiser R. I. Angew. Chem., Int. Ed. 2019;58:5663. doi: 10.1002/anie.201901059. - DOI - PubMed
- Bernhardt H. S. Life. 2019;9:19. doi: 10.3390/life9010019. - DOI - PMC - PubMed
-
-
-
For recent reviews:
- Reckenthäler M. Griesbeck A. G. Adv. Synth. Catal. 2013;355:2727. doi: 10.1002/adsc.201300751. - DOI
- Xuan J. Lu L.-Q. Chen J.-R. Xiao W.-J. Eur. J. Org. Chem. 2013;2013:6755. doi: 10.1002/ejoc.201300596. - DOI
- Koike T. Akita M. Top. Organomet. Chem. 2014;48:371. doi: 10.1007/3418_2014_80. - DOI
- Wang C. Lu Z. Org. Chem. Front. 2015;2:179. doi: 10.1039/C4QO00306C. - DOI
- Aukauloo A. and Leibl W., Chapter Three - Bioinspired Photocatalysis, Advances in Botanical Research, ed. B. Robert, Academic Press, 2016, vol. 79, p. 63
- Kokotos C. G. Org. Biomol. Chem. 2018;16:4596. doi: 10.1039/C8OB00725J. - DOI - PubMed
- Zeitler K., Metal-free Photo (redox) Catalysis, Visible Light Photocatalysis in Organic Chemistry, ed. C. R. J. Stephenson, T. P. Yoon and D. W. C. MacMillan, Wiley-VCH, Weinheim, 2018
- Zhou Q.-Q. Zou Y.-Q. Lu L.-Q. Xiao W.-J. Angew. Chem., Int. Ed. 2019;58:1586. doi: 10.1002/anie.201803102. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources

