Spectroscopic and chromatographic investigation of chiral interactions between tiaprofenic acid and alginate-metal-complexes
- PMID: 35515692
- PMCID: PMC9056893
- DOI: 10.1039/d0ra07665a
Spectroscopic and chromatographic investigation of chiral interactions between tiaprofenic acid and alginate-metal-complexes
Abstract
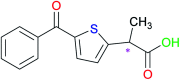
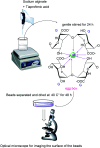
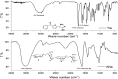
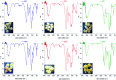
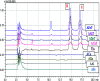
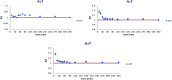
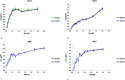
Alginate-metal-complexes of Ca, Ba, Zn, Fe and Al were prepared in the form of beads. The preparation was based on the ionotropic method to obtain blank beads (unloaded) and beads loaded with tiaprofenic acid. IR spectra of blank beads, drug loaded beads and physical mixtures of the drug with the blank beads were recorded. The comparison of these spectra, especially in the region of hydroxyl group, implied chiral interactions between the drug and the complexes. Additionally, the drug was released from the loaded beads in aqueous phosphate buffer solutions (PBS) at pH = 7.4. Chiral HPLC was used to determine the enantiomeric excess, % ee, of the released drug. The determined % ee values indicated chiral interactions between tiaprofenic acid and alginate-metal-complexes. However, various mathematical models were used to simulate the release kinetics for each enantiomer. The metal content of Na, Ca, Ba, Zn, Fe and Al in the studied materials was measured using atomic absorption spectroscopy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Reddy I. K. and Mehva R., Chirality in Drug Design and Development, USA, 2004
-
- Ananthi N. Organic & Medicinal Chemistry International Journal. 2018;5:1–6.
-
- Yu H. Yong X. Liang J. Deng J. Wu Y. Ind. Eng. Chem. Res. 2016;55:6037–6048. doi: 10.1021/acs.iecr.6b01031. - DOI
-
- Gohel M. C. Dissolution Technol. 2003;10:16–20. doi: 10.14227/DT100303P16. - DOI
-
- Mohan S. J. Mohan E. C. Yamsani M. R. Int. J. Pharm. Sci. Nanotechnol. 2009;1:309–316.
LinkOut - more resources
Full Text Sources

