Systematic overview of soft materials as a novel frontier for MRI contrast agents
- PMID: 35515779
- PMCID: PMC9055484
- DOI: 10.1039/d0ra03194a
Systematic overview of soft materials as a novel frontier for MRI contrast agents
Abstract
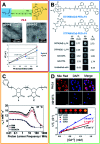
Magnetic resonance imaging (MRI) is a well-known diagnostic technique used to obtain high quality images in a non-invasive manner. In order to increase the contrast between normal and pathological regions in the human body, positive (T1) or negative (T2) contrast agents (CAs) are commonly intravenously administered. The most efficient class of T1-CAs are based on kinetically stable and thermodynamically inert gadolinium complexes. In the last two decades many novel macro- and supramolecular CAs have been proposed. These approaches have been optimized to increase the performance of the CAs in terms of the relaxivity values and to reduce the administered dose, decreasing the toxicity and giving better safety and pharmacokinetic profiles. The improved performances may also allow further information to be gained on the pathological and physiological state of the human body. The goal of this review is to report a systematic overview of the nanostructurated CAs obtained and developed by manipulating soft materials at the nanometer scale. Specifically, our attention is centered on recent examples of fibers, hydrogels and nanogel formulations, that seem particularly promising for overcoming the problematic issues that have recently pushed the European Medicines Agency (EMA) to withdraw linear CAs from the market.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Dual T1/ T2 Nanoscale Coordination Polymers as Novel Contrast Agents for MRI: A Preclinical Study for Brain Tumor.ACS Appl Mater Interfaces. 2018 Nov 14;10(45):38819-38832. doi: 10.1021/acsami.8b15594. Epub 2018 Nov 1. ACS Appl Mater Interfaces. 2018. PMID: 30351897
-
Toxicity of magnetic resonance imaging agents: small molecule and nanoparticle.Curr Top Med Chem. 2013;13(4):434-45. doi: 10.2174/1568026611313040004. Curr Top Med Chem. 2013. PMID: 23432006 Review.
-
Contrast agents: magnetic resonance.Handb Exp Pharmacol. 2008;(185 Pt 1):135-65. doi: 10.1007/978-3-540-72718-7_7. Handb Exp Pharmacol. 2008. PMID: 18626802 Review.
-
Chemistry of paramagnetic and diamagnetic contrast agents for Magnetic Resonance Imaging and Spectroscopy pH responsive contrast agents.Eur J Radiol. 2008 Sep;67(3):453-8. doi: 10.1016/j.ejrad.2008.02.048. Epub 2008 May 1. Eur J Radiol. 2008. PMID: 18455343 Review.
-
Mesoporous manganese silicate coated silica nanoparticles as multi-stimuli-responsive T1-MRI contrast agents and drug delivery carriers.Acta Biomater. 2016 Jan;30:378-387. doi: 10.1016/j.actbio.2015.11.036. Epub 2015 Nov 18. Acta Biomater. 2016. PMID: 26602820
Cited by
-
Self-Assembled Block Copolymers as a Facile Pathway to Create Functional Nanobiosensor and Nanobiomaterial Surfaces.Polymers (Basel). 2024 May 1;16(9):1267. doi: 10.3390/polym16091267. Polymers (Basel). 2024. PMID: 38732737 Free PMC article. Review.
-
Toxicity Mechanisms of Gadolinium and Gadolinium-Based Contrast Agents-A Review.Int J Mol Sci. 2024 Apr 6;25(7):4071. doi: 10.3390/ijms25074071. Int J Mol Sci. 2024. PMID: 38612881 Free PMC article. Review.
-
Evaluating the use of lanthanide containing dendrimers for solvent paramagnetic relaxation enhancement.J Biomol NMR. 2025 Sep;79(3):199-208. doi: 10.1007/s10858-025-00468-9. Epub 2025 Apr 10. J Biomol NMR. 2025. PMID: 40208391 Free PMC article.
-
Peptide-Hydrogel Nanocomposites for Anti-Cancer Drug Delivery.Gels. 2023 Dec 4;9(12):953. doi: 10.3390/gels9120953. Gels. 2023. PMID: 38131939 Free PMC article. Review.
-
In vivo near-infrared fluorescent fibrin highlights growth of nerve during regeneration across a nerve gap.J Biomed Opt. 2022 Jul;27(7):070502. doi: 10.1117/1.JBO.27.7.070502. Epub 2022 Jul 20. J Biomed Opt. 2022. PMID: 36451699 Free PMC article.
References
-
- Salzer R., Biomedical imaging: principles and applications, John Wiley & Sons, Inc., Hoboken, NJ USA, 2012
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

