Nanostructured N doped TiO2 efficient stable catalyst for Kabachnik-Fields reaction under microwave irradiation
- PMID: 35515785
- PMCID: PMC9055502
- DOI: 10.1039/d0ra04533k
Nanostructured N doped TiO2 efficient stable catalyst for Kabachnik-Fields reaction under microwave irradiation
Abstract
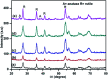
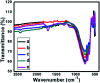
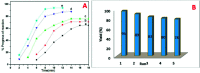
Herein, we report nitrogen-doped TiO2 (N-TiO2) solid-acid nanocatalysts with heterogeneous structure employed for the solvent-free synthesis of α-aminophosphonates through Kabachnik-Fields reaction. N-TiO2 were synthesized by direct amination using triethylamine as a source of nitrogen at low temperature and optimized by varying the volume ratios of TiCl4, methanol, water, and triethylamine, under identical conditions. An X-ray diffraction (XRD) study showed the formation of a rutile phase and the crystalline size is 10 nm. The nanostructural features of N-TiO2 were examined by HR-TEM analysis, which showed they had rod-like morphology with a diameter of ∼7 to 10 nm. Diffuse reflectance spectra show the extended absorbance in the visible region with a narrowing in the band gap of 2.85 eV, and the high resolution XPS spectrum of the N 1s region confirmed successful doping of N in the TiO2 lattice. More significantly, we found that as-synthesized N-TiO2 showed significantly higher catalytic activity than commercially available TiO2 for the synthesis of a novel series of α-amino phosphonates via Kabachnik-Fields reaction under microwave irradiation conditions. The improved catalytic activity is due to the presence of strong and Bronsted acid sites on a porous nanorod surface. This work signifies N-TiO2 is an efficient stable catalyst for the synthesis of α-aminophosphonate derivatives.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









Similar articles
-
Enhanced photo-catalytic activity of Sr and Ag co-doped TiO2 nanoparticles for the degradation of Direct Green-6 and Reactive Blue-160 under UV & visible light.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Oct 5;149:571-9. doi: 10.1016/j.saa.2015.04.101. Epub 2015 May 8. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25983059
-
[Spectral Characteristics and Catalytic Performances of SO2-4/Ce-TiO2 with Visible Light Response].Guang Pu Xue Yu Guang Pu Fen Xi. 2016 Apr;36(4):1133-8. Guang Pu Xue Yu Guang Pu Fen Xi. 2016. PMID: 30052013 Chinese.
-
Microstructures and optical performances of nitrogen-vanadium co-doped TiO2 with enhanced purification efficiency to vehicle exhaust.Environ Res. 2021 Feb;193:110560. doi: 10.1016/j.envres.2020.110560. Epub 2020 Dec 8. Environ Res. 2021. PMID: 33279493
-
Synergistic effect of N- and F-codoping on the structure and photocatalytic performance of TiO2.J Environ Sci (China). 2015 Feb 1;28:148-56. doi: 10.1016/j.jes.2014.06.046. Epub 2014 Dec 13. J Environ Sci (China). 2015. PMID: 25662249 Review.
-
Synthesis of α-Aminophosphonates and Related Derivatives; the Last Decade of the Kabachnik-Fields Reaction.Molecules. 2021 Apr 25;26(9):2511. doi: 10.3390/molecules26092511. Molecules. 2021. PMID: 33923090 Free PMC article. Review.
Cited by
-
Enzymatic Synthesis of a Novel Coumarin Aminophosphonates: Antibacterial Effects and Oxidative Stress Modulation on Selected E. coli Strains.Int J Mol Sci. 2023 Apr 20;24(8):7609. doi: 10.3390/ijms24087609. Int J Mol Sci. 2023. PMID: 37108774 Free PMC article.
-
Recent Advances in the Application of P(III)-Nucleophiles to Create New P-C Bonds through Michaelis-Arbuzov-Type Rearrangement.Top Curr Chem (Cham). 2024 Mar 8;382(1):10. doi: 10.1007/s41061-024-00456-x. Top Curr Chem (Cham). 2024. PMID: 38457062 Review.
-
AlCl3@ZnO nanostructured material: an efficient green catalyst for the one-pot solvent-free synthesis of 1,4-dihydropyridines.RSC Adv. 2023 Aug 18;13(35):24767-24776. doi: 10.1039/d3ra04277d. eCollection 2023 Aug 11. RSC Adv. 2023. PMID: 37601590 Free PMC article.
-
Ni loaded SnS2 hexagonal nanosheets for photocatalytic hydrogen generation via water splitting.RSC Adv. 2023 Jan 16;13(4):2418-2426. doi: 10.1039/d2ra07954b. eCollection 2023 Jan 11. RSC Adv. 2023. PMID: 36741188 Free PMC article.
-
Improvement of low-temperature NH3-SCR catalytic performance over nitrogen-doped MO x -Cr2O3-La2O3/TiO2-N (M = Cu, Fe, Ce) catalysts.RSC Adv. 2021 Jun 28;11(37):22780-22788. doi: 10.1039/d1ra03845a. eCollection 2021 Jun 25. RSC Adv. 2021. PMID: 35480444 Free PMC article.
References
-
- Eto M., Organophosphorus pesticides, CRC press, 2018
-
- Azaam M. M. Kenawy E. R. El-din A. S. Khamis A. A. El-Magd M. A. J. Saudi Chem. Soc. 2018;22(1):34–41. doi: 10.1016/j.jscs.2017.06.002. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

