The caffeic acid moiety plays an essential role in attenuating lipid accumulation by chlorogenic acid and its analogues
- PMID: 35515874
- PMCID: PMC9063487
- DOI: 10.1039/c8ra09395d
The caffeic acid moiety plays an essential role in attenuating lipid accumulation by chlorogenic acid and its analogues
Abstract
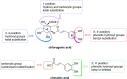
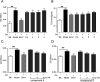
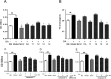
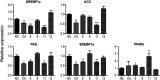
Chlorogenic acid (5-caffeoylquinic, CA) possesses distinct hypolipidemic properties in vivo and in vitro, yet the structure-activity relationship (SAR) of CA on lipid metabolism remains unknown. To achieve this aim, we designed and synthesized two sets of CA analogues and evaluated their efficacies to prevent oleic acid (OA)-elicited lipid accumulation in HepG2 cells. Blockage of all hydroxyl and carboxyl groups on the quinic acid moiety did not deteriorate the hypolipidemic effect of CA while blockage of all phenolic hydroxyl groups on the caffeic acid moiety abolished the activity of CA. Further replacement of the quinic acid moiety with cyclohexane and modification of individual phenolic hydroxyl groups on the caffeic acid moiety showed that the phenolic-hydroxyl-reserved analogues displayed a more potent hypolipidemic effect than CA, whereas the analogue with no phenolic hydroxyl displayed little effect on the OA-elicited lipid accumulation. In accordance, the modulating effects of CA on the transcription of the lipogenic gene sterol-regulatory element binding protein (SREBP)1c/1a, acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS) and peroxisome proliferator-activated receptor α (PPARα) were also abolished when the phenolic hydroxyl groups on the caffeic acid moiety were blocked. Our results suggest that the phenolic hydroxyl on the caffeic acid moiety is vital for the lipid-lowering activity of CA.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
We declare there are no financial or other contractual agreements that might cause conflicts of interest or be perceived as causing conflicts of interest.
Figures
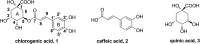
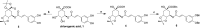
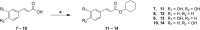
Similar articles
-
Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPARα and SREBP-1.Food Chem Toxicol. 2013 Aug;58:198-209. doi: 10.1016/j.fct.2013.04.018. Epub 2013 Apr 18. Food Chem Toxicol. 2013. PMID: 23603006
-
Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice.Food Chem Toxicol. 2014 Feb;64:94-103. doi: 10.1016/j.fct.2013.11.015. Epub 2013 Nov 22. Food Chem Toxicol. 2014. PMID: 24275089
-
Melatonin Modulates lipid Metabolism in HepG2 Cells Cultured in High Concentrations of Oleic Acid: AMPK Pathway Activation may Play an Important Role.Cell Biochem Biophys. 2018 Dec;76(4):463-470. doi: 10.1007/s12013-018-0859-0. Epub 2018 Oct 15. Cell Biochem Biophys. 2018. PMID: 30324559
-
Sasa quelpaertensis and p-coumaric acid attenuate oleic acid-induced lipid accumulation in HepG2 cells.Biosci Biotechnol Biochem. 2013;77(7):1595-8. doi: 10.1271/bbb.130167. Epub 2013 Jul 7. Biosci Biotechnol Biochem. 2013. PMID: 23832345
-
Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells.BMC Complement Altern Med. 2019 Sep 13;19(1):255. doi: 10.1186/s12906-019-2671-9. BMC Complement Altern Med. 2019. PMID: 31519174 Free PMC article.
Cited by
-
Chlorogenic Acid Derivatives: Structural Modifications, Drug Design, and Biological Activities: A Review.Mini Rev Med Chem. 2024;24(7):748-766. doi: 10.2174/1389557523666230822095959. Mini Rev Med Chem. 2024. PMID: 37608658 Review.
-
Cinnamic acid ameliorate gentamicin-induced liver dysfunctions and nephrotoxicity in rats through induction of antioxidant activities.Heliyon. 2021 Jul 2;7(7):e07465. doi: 10.1016/j.heliyon.2021.e07465. eCollection 2021 Jul. Heliyon. 2021. PMID: 34278037 Free PMC article.
-
Amino acid ester-coupled caffeoylquinic acid derivatives as potential hypolipidemic agents: synthesis and biological evaluation.RSC Adv. 2021 Jan 5;11(3):1654-1661. doi: 10.1039/d0ra09621k. eCollection 2021 Jan 4. RSC Adv. 2021. PMID: 35424091 Free PMC article.
References
-
- Karr S. Am. J. Manag. Care. 2017;23:S139–S148. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

