Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection
- PMID: 35515907
- PMCID: PMC9060762
- DOI: 10.1039/c8ra09157a
Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection
Abstract
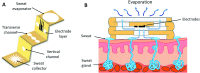
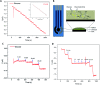
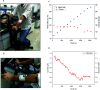
Wearable electrochemical sensors have attracted tremendous attention in recent years. Here, a three-dimensional paper-based microfluidic electrochemical integrated device (3D-PMED) was demonstrated for real-time monitoring of sweat metabolites. The 3D-PMED was fabricated by wax screen-printing patterns on cellulose paper and then folding the pre-patterned paper four times to form five stacked layers: the sweat collector, vertical channel, transverse channel, electrode layer and sweat evaporator. A sweat monitoring device was realized by integrating a screen-printed glucose sensor on polyethylene terephthalate (PET) substrate with the fabricated 3D-PMED. The sweat flow process in 3D-PMED was modelled with red ink to demonstrate the capability of collecting, analyzing and evaporating sweat, due to the capillary action of filter paper and hydrophobicity of wax. The glucose sensor was designed with a high sensitivity (35.7 μA mM-1 cm-2) and low detection limit (5 μM), considering the low concentration of glucose in sweat. An on-body experiment was carried out to validate the practicability of the three-dimensional sweat monitoring device. Such a 3D-PMED can be readily expanded for the simultaneous monitoring of alternative sweat electrolytes and metabolites.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Kim D.-H. Lu N. Ma R. Kim Y.-S. Kim R.-H. Wang S. Wu J. Won S. M. Tao H. Islam A. Yu K. J. Kim T.-i. Chowdhury R. Ying M. Xu L. Li M. Chung H.-J. Keum H. McCormick M. Liu P. Zhang Y.-W. Omenetto F. G. Huang Y. Coleman T. Rogers J. A. Science. 2011;333:838–843. doi: 10.1126/science.1206157. - DOI - PubMed
-
- Bariya M. Nyein H. Y. Y. Javey A. Nature Electronics. 2018;1:160–171. doi: 10.1038/s41928-018-0043-y. - DOI
-
- Mukhopadhyay S. C. IEEE Sens. J. 2015;15:1321–1330.
LinkOut - more resources
Full Text Sources
Other Literature Sources

