Mussel-inspired nano-silver loaded layered double hydroxides embedded into a biodegradable polymer matrix for enhanced mechanical and gas barrier properties
- PMID: 35515932
- PMCID: PMC9060778
- DOI: 10.1039/c8ra09602c
Mussel-inspired nano-silver loaded layered double hydroxides embedded into a biodegradable polymer matrix for enhanced mechanical and gas barrier properties
Abstract
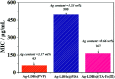
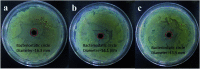
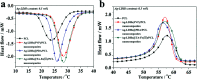
In this paper, a facile, green and mussel-inspired method is presented to prepare silver loaded layered double hydroxides (Ag-LDHs@PDA and Ag-LDHs@TA-Fe(iii)) using a pre-synthesis polydopamine (PDA)/tannic acid (TA)-Fe(iii) layer as a nanoscale guide and PDA/TA itself as a reducing reagent to form uniform silver nanoparticles (AgNPs) on the surface of modified LDHs. Meanwhile, another kind of LDH, Ag-LDHs(PVP), was prepared via the direct reduction of the precursor [Ag(NH3)2]+ with polyvinyl pyrrolidone (PVP). And three kinds of Ag-LDHs/poly(ε-caprolactone) (PCL) nanocomposite were prepared by blending Ag-LDHs and pure PCL via a solution casting method to obtain homogeneous films. It is shown that the obtained AgNPs are distributed on the LDH surfaces uniformly. And the high loading and medium size of the AgNPs present in Ag-LDHs(PVP) give it the best antibacterial properties. However, compared with Ag-LDHs(PVP), the better dispersibilities of Ag-LDHs@PDA and Ag-LDHs@TA-Fe(iii) contribute to the greater aspect ratios of Ag-LDHs in the matrices, resulting in an increase in the number of tortuous paths for gas diffusion. Meanwhile, Ag-LDHs@PDA and Ag-LDHs@TA-Fe(iii) have stronger interactions with the PCL matrix, which is favorable for the existence of less interface defects in the matrix, resulting in an improvement in the mechanical and gas barrier properties. Therefore, mussel-inspired antibacterial Ag-LDHs/PCL nanocomposites show preferable mechanical and gas barrier properties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures









References
-
- Zhao S. Li J. Silver-Cobalt Oxides Derived from Silver Nanoparticles Deposited on Layered Double Hydroxides for Methane Combustion. ChemCatChem. 2015;7(13):1966–1974. doi: 10.1002/cctc.201500254. - DOI
-
- Leng J. Kang N. Wang D. et al., Structure-Property Relationships of Nanocomposites Based on Polylactide and Layered Double Hydroxides – Comparison of MgAl and NiAl LDH as Nanofiller. Macromol. Chem. Phys. 2017;218(20):1700232. doi: 10.1002/macp.201700232. - DOI
-
- Allou N. G. B. Saikia P. Borah A. et al., Hybrid nanocomposites of layered double hydroxides: an update of their biological applications and future prospects. Colloid Polym. Sci. 2017;295(5):725–747. doi: 10.1007/s00396-017-4047-3. - DOI
-
- Wang C. Han H. Jiang W. et al., Immobilization of Thermostable Lipase QLM on Core-Shell Structured Polydopamine-Coated Fe3O4 Nanoparticles. Catalysts. 2017;7(2):49–60. doi: 10.3390/catal7020049. - DOI
-
- Nam H. J. Park E. B. Jung D. Bioinspired polydopamine-layered double hydroxide nanocomposites: controlled synthesis and multifunctional performance. RSC Adv. 2016;6(30):24952–24958. doi: 10.1039/C5RA28103B. - DOI
LinkOut - more resources
Full Text Sources

