Preparation and photophysical properties of quinazoline-based fluorophores
- PMID: 35516009
- PMCID: PMC9056281
- DOI: 10.1039/d0ra05701k
Preparation and photophysical properties of quinazoline-based fluorophores
Abstract
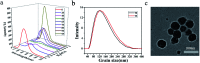
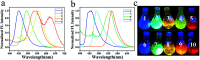
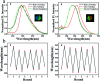
The donor-acceptor design is a classic method of synthesizing new fluorescent molecules. In this study, a series of new fluorescent compounds (1-10) were synthesized based on 2-(3,5-bis(trifluoromethyl)phenyl)-quinazoline acceptor and various amino donors. The fluorescent emissions of 1-10 cover the spectrum from 414 nm to 597 nm in cyclohexane solutions with various amino donors on 4- or 7-positions of quinazoline. Ultimately, compounds 1 and 2 presented the highest photoluminescence quantum yield (QY) over 80%, while compound 10 provided the largest Stokes shift (161 nm) in cyclohexane. Most of them have strong emissions in aggregated states such as in nanoparticles, in powders, in crystals and in films. Mechanochromic properties were observed for compounds 1, 2, 4 and 7. Furthermore, blue OLEDs were fabricated by using compound 2 or 7 as the active layer.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Efficient orange-red electroluminescence of iridium complexes with 1-(2,6-bis(trifluoromethyl)pyridin-4-yl)isoquinoline and 4-(2,6-bis(trifluoromethyl)pyridin-4-yl)quinazoline ligands.Dalton Trans. 2017 Nov 7;46(43):14916-14925. doi: 10.1039/c7dt03310a. Dalton Trans. 2017. PMID: 29043334
-
Phenothiazine versus Phenoxazine: Structural Effects on the Photophysical Properties of NIR-II AIE Fluorophores.ACS Appl Mater Interfaces. 2020 Sep 30;12(39):43466-43473. doi: 10.1021/acsami.0c12773. Epub 2020 Sep 21. ACS Appl Mater Interfaces. 2020. PMID: 32907323
-
Carbazole donor-carbazole linker-based compounds: preparation, photophysical properties, and formation of fluorescent nanoparticles.J Phys Chem A. 2010 Apr 8;114(13):4550-7. doi: 10.1021/jp912286u. J Phys Chem A. 2010. PMID: 20232882
-
Influence of Structural Isomerism on the Photophysical Properties of a Series of Donor-Acceptor 1-Naphthalenecarbonitrile Derivatives Possessing Amine Substituents.J Phys Chem A. 2020 Mar 19;124(11):2113-2122. doi: 10.1021/acs.jpca.9b10788. Epub 2020 Mar 5. J Phys Chem A. 2020. PMID: 32068405
-
Syntheses, photoluminescence, and electroluminescence of a series of iridium complexes with trifluoromethyl-substituted 2-phenylpyridine as the main ligands and tetraphenylimidodiphosphinate as the ancillary ligand.Inorg Chem. 2013 May 6;52(9):4916-25. doi: 10.1021/ic302510p. Epub 2013 Apr 15. Inorg Chem. 2013. PMID: 23586330
Cited by
-
Understanding the aqueous chemistry of quinoline and the diazanaphthalenes: insight from DFT study.Heliyon. 2021 Jul 10;7(7):e07531. doi: 10.1016/j.heliyon.2021.e07531. eCollection 2021 Jul. Heliyon. 2021. PMID: 34296019 Free PMC article.
-
Design, Synthesis, and Photophysical Properties of 5-Aminobiphenyl Substituted [1,2,4]Triazolo[4,3-c]- and [1,2,4]Triazolo[1,5-c]quinazolines.Molecules. 2024 May 24;29(11):2497. doi: 10.3390/molecules29112497. Molecules. 2024. PMID: 38893371 Free PMC article.
-
Tetraphenylethene-Modified Colorimetric and Fluorescent Chemosensor for Hg2+ With Aggregation-Induced Emission Enhancement, Solvatochromic, and Mechanochromic Fluorescence Features.Front Chem. 2022 Jan 26;9:811294. doi: 10.3389/fchem.2021.811294. eCollection 2021. Front Chem. 2022. PMID: 35155382 Free PMC article.
-
Push-Pull Structures Based on 2-Aryl/thienyl Substituted Quinazolin-4(3H)-ones and 4-Cyanoquinazolines.Molecules. 2022 Oct 22;27(21):7156. doi: 10.3390/molecules27217156. Molecules. 2022. PMID: 36363984 Free PMC article.
-
A Turn-On Quinazolinone-Based Fluorescence Probe for Selective Detection of Carbon Monoxide.Molecules. 2023 Apr 22;28(9):3654. doi: 10.3390/molecules28093654. Molecules. 2023. PMID: 37175064 Free PMC article.
References
-
- Ma Z.-Z. Hano Y. Nomura T. Chen Y.-J. Heterocycles. 1997:541–546.
LinkOut - more resources
Full Text Sources
Miscellaneous

