A deep-red fluorescent molecular rotor based on donor-two-acceptor modular system for imaging mitochondrial viscosity
- PMID: 35516013
- PMCID: PMC9056405
- DOI: 10.1039/d0ra04935b
A deep-red fluorescent molecular rotor based on donor-two-acceptor modular system for imaging mitochondrial viscosity
Abstract
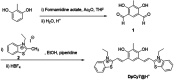
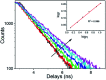
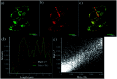
A new donor-two-acceptor modular fluorescence rotor DpCy7 involving a phenolate donor unit and two benzothiazolium acceptor moieties was designed and synthesized. The DpCy7 underwent an internal charge transfer to form a Cy7-like longer conjugated system fluorochrome at a physiological pH. The probe exhibited a strong turn-on (8.5-fold) deep-red emission with a larger Stokes shift in glycerol aqueous solutions with restriction of rotation. Both the fluorescence intensity and fluorescence lifetime displayed the linear relationship of viscosity changes in the logarithmic plots. Furthermore, the HeLa cell imaging experiments of DpCy7 indicated that the rotor could be used to monitor the mitochondrial viscosity in living cells. This new type of deep-red fluorescence rotor provides a potential platform for determining viscosity at subcellular levels.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
LinkOut - more resources
Full Text Sources

