Phenylboronic acid derivative-modified (6,5) single-wall carbon nanotube probes for detecting glucose and hydrogen peroxide
- PMID: 35516097
- PMCID: PMC9059849
- DOI: 10.1039/c8ra09272a
Phenylboronic acid derivative-modified (6,5) single-wall carbon nanotube probes for detecting glucose and hydrogen peroxide
Abstract
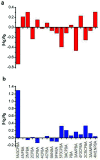
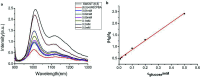
In this paper, we presented a new method for constructing near-infrared fluorescence probes and their applications in detecting glucose and hydrogen peroxide (H2O2). We used purified (6,5) single-wall carbon nanotubes (SWCNTs) separated by a polyethylene glycol/dextran aqueous two-phase system as the basis for near-infrared probes. Different phenylboronic acids were used for non-covalent modification of SWCNTs (6,5). Glucose was detected by the specific binding of the boronic acid group with cis-diol. Hydrogen peroxide was detected by horseradish peroxidase (HRP) combined with phenylboronic-acid-modified SWCNTs. The results revealed that the fluorescence intensity of purified SWCNTs was significantly enhanced without other chiral nanotube interactions compared to that of the raw SWCNT material. The fluorescence responses of 3-carboxy-5-nitrophenylboronic acid-modified purified CNTs could be used to effectively measure glucose in the concentration range from 0.01 mM to 0.50 mM with an interval linear index of R 2 = 0.996 (LOD = 1.7 μM, S/N = 3). The detected H2O2 with the 3-aminobenzeneboronic acid-modified (6,5) SWCNT with HRP was in the concentration range from 5.0 μM to 40 μM with an interval linear index of R 2 = 0.997 (LOD = 0.85 μM, S/N = 3). Moreover, this sensor exhibited a strong anti-interference effect without biological matrix fluorescence effects above 1000 nm wavelength. Thus, the proposed novel near-infrared fluorescence probes employed for reducing the interference of the biomatrix in this region are expected to be used in subsequent biosensor investigations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







Similar articles
-
Utilization of highly purified single wall carbon nanotubes dispersed in polymer thin films for an improved performance of an electrochemical glucose sensor.Mater Sci Eng C Mater Biol Appl. 2014 Jul 1;40:299-307. doi: 10.1016/j.msec.2014.04.009. Epub 2014 Apr 13. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 24857497
-
Direct and mediated electrochemistry of peroxidase and its electrocatalysis on a variety of screen-printed carbon electrodes: amperometric hydrogen peroxide and phenols biosensor.Anal Bioanal Chem. 2015 Jan;407(2):439-46. doi: 10.1007/s00216-014-8282-x. Epub 2014 Nov 6. Anal Bioanal Chem. 2015. PMID: 25374125
-
Peroxide Electrochemical Sensor and Biosensor Based on Nanocomposite of TiO2 Nanoparticle/Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode.Nanomaterials (Basel). 2019 Dec 27;10(1):64. doi: 10.3390/nano10010064. Nanomaterials (Basel). 2019. PMID: 31892125 Free PMC article.
-
Improving stability of biosensor based on covalent immobilization of horseradish peroxidase by γ-aminobutyric acid and application in detection of H2O2.Int J Biol Macromol. 2019 Sep 1;136:597-606. doi: 10.1016/j.ijbiomac.2019.06.103. Epub 2019 Jun 15. Int J Biol Macromol. 2019. PMID: 31212047
-
Diol or Hydrogen Peroxide-responsive Micellar Systems and Their Rheological Properties.J Oleo Sci. 2024;73(4):611-618. doi: 10.5650/jos.ess22420. J Oleo Sci. 2024. PMID: 38556294 Review.
Cited by
-
Advances in the Design of Phenylboronic Acid-Based Glucose-Sensitive Hydrogels.Polymers (Basel). 2023 Jan 23;15(3):582. doi: 10.3390/polym15030582. Polymers (Basel). 2023. PMID: 36771883 Free PMC article. Review.
-
Enhancing Intracellular Optical Performance and Stability of Engineered Nanomaterials via Aqueous Two-Phase Purification.Nano Lett. 2023 Jul 26;23(14):6588-6595. doi: 10.1021/acs.nanolett.3c01727. Epub 2023 Jul 6. Nano Lett. 2023. PMID: 37410951 Free PMC article.
References
LinkOut - more resources
Full Text Sources

