Computational study on the mechanisms and kinetics of the CH2BrO2 + ClO reaction in the atmosphere
- PMID: 35516180
- PMCID: PMC9055081
- DOI: 10.1039/c9ra10511e
Computational study on the mechanisms and kinetics of the CH2BrO2 + ClO reaction in the atmosphere
Abstract
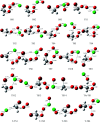
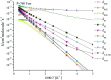
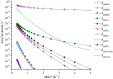
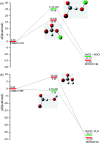
The singlet and triplet potential energy surfaces for the CH2BrO2 + ClO reaction are studied at the CCSD(T)/cc-pVTZ//B3LYP/6-311++G(d,p) level. CH2BrO2 is revealed to react with ClO through two kinds of mechanisms on the triplet potential energy surface (PES), namely, SN2 displacement and H-abstraction, and the production of P3 (CHBrO2 + HOCl) via H-abstraction is the dominant channel. Addition/elimination and SN2 displacement mechanisms exist on the singlet PES and are more complicated. The RRKM calculations of the mechanism and product distribution in the CH2BrO2 + ClO reaction show that the stabilization of IM1 (CH2BrOOOBr) is dominant at T ≤ 600 K, while the pathway of producing P1 (CHBrO + HO2 + Cl) occupies the entire reaction at T > 600 K. The total rate constants are independent of pressure, while the individual rate constants are sensitive to pressure. The lifetime of CH2BrO2 in the presence of ClO is estimated to be 20.27 h. Moreover, time-dependent density functional theory (TDDFT) calculations suggest that IM1 (CH2BrOOOCl), IM2 (CH2BrOOClO) and IM3 (CH2BrOClO2) will photolyze under the sunlight.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Finlayson-Pitts B. and Pitts Jr J. N., Chemistry of the Upper and Lower Atmosphere, Academic Press, New York, 1999
-
- Wayne R. P., Chemistry of Atmospheres, Oxford University Press, Oxford, 3rd edn, 2000
-
- Albritton D. L. and Watson R. T., Methyl Bromide: Its Atmospheric Science, Technology and Economics, Montreal Protocol Assessment Supplement. UNEP, Nairobi, Kenya, 1993
-
- Montzka S. A. and Reimann S., et al., Ozone-depleting substances (ODSs) and related chemicals. Scientific Assessment of Ozone Depletion: 2010, Global Ozone Research and Monitoring Project Report No. 52, World Meteorological Organization, Geneva, Switzerland, ch. 1, 2011
-
- McGivern W. S. Kim H. Francisco J. S. North S. W. J. Phys. Chem. A. 2004;108:7247–7252. doi: 10.1021/jp0311613. - DOI
LinkOut - more resources
Full Text Sources

