Disulfide based prodrugs for cancer therapy
- PMID: 35516223
- PMCID: PMC9055211
- DOI: 10.1039/d0ra04155f
Disulfide based prodrugs for cancer therapy
Abstract
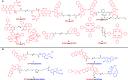
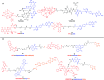
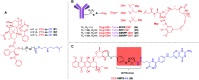
Advances in the tumor microenvironment have facilitated the development of novel anticancer drugs and delivery vehicles for improved therapeutic efficacy and decreased side effects. Disulfide bonds with unique chemical and biophysical properties can be used as cleavable linkers for the delivery of chemotherapeutic drugs. Accordingly, small molecule-, peptide-, polymer- and protein-based multifunctional prodrugs bearing cleavable disulfide bonds are well accepted in clinical settings. Herein, we first briefly introduce a number of prodrugs and divide them into three categories, namely, disulfide-containing small molecule conjugates, disulfide-containing cytotoxic agent-targeted fluorescent agent conjugates, and disulfide-containing cytotoxic agent-macromolecule conjugates. Then, we discuss the complex redox environment and the underlying mechanism of free drug release from disulfide based prodrugs in in vivo settings. Based on these insights, we analyze the impact of electronics, steric hindrance and substituent position of the disulfide linker on the extracellular stability and intracellular cleavage rate of disulfide containing prodrugs. Current challenges and future opportunities for the disulfide linker are provided at the end.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




Similar articles
-
Disulfide-based multifunctional conjugates for targeted theranostic drug delivery.Acc Chem Res. 2015 Nov 17;48(11):2935-46. doi: 10.1021/acs.accounts.5b00406. Epub 2015 Oct 29. Acc Chem Res. 2015. PMID: 26513450
-
Synthesis and biological evaluation of antibody conjugates of phosphate prodrugs of cytotoxic DNA alkylators for the targeted treatment of cancer.J Med Chem. 2012 Jan 26;55(2):766-82. doi: 10.1021/jm201284m. Epub 2012 Jan 5. J Med Chem. 2012. PMID: 22148292
-
Mutual prodrugs containing bio-cleavable and drug releasable disulfide linkers.Bioorg Chem. 2013 Aug;49:40-8. doi: 10.1016/j.bioorg.2013.06.007. Epub 2013 Jul 3. Bioorg Chem. 2013. PMID: 23886697
-
Targeted cancer therapy: conferring specificity to cytotoxic drugs.Acc Chem Res. 2008 Jan;41(1):98-107. doi: 10.1021/ar700108g. Epub 2007 Aug 18. Acc Chem Res. 2008. PMID: 17705444 Review.
-
Drug Conjugates Using Different Dynamic Covalent Bonds and their Application in Cancer Therapy.Curr Drug Deliv. 2020;17(7):542-557. doi: 10.2174/1567201817999200508092141. Curr Drug Deliv. 2020. PMID: 32384029 Review.
Cited by
-
Boronic acid based dynamic click chemistry: recent advances and emergent applications.Chem Sci. 2020 Dec 17;12(5):1585-1599. doi: 10.1039/d0sc05009a. Chem Sci. 2020. PMID: 34163920 Free PMC article. Review.
-
Stabilized Carbon Radical-Mediated Assembly of Arylthianthrenium Salts, Alkenes and Amino Acid/Peptide Derivatives.Adv Sci (Weinh). 2025 Jan;12(2):e2411579. doi: 10.1002/advs.202411579. Epub 2024 Nov 21. Adv Sci (Weinh). 2025. PMID: 39573977 Free PMC article.
-
Advance Progress in Assembly Mechanisms of Carrier-Free Nanodrugs for Cancer Treatment.Molecules. 2023 Oct 13;28(20):7065. doi: 10.3390/molecules28207065. Molecules. 2023. PMID: 37894544 Free PMC article. Review.
-
Activatable theranostic prodrug scaffold with tunable drug release rate for sequential photodynamic and chemotherapy.Smart Mol. 2024 Feb 20;2(1):e20230024. doi: 10.1002/smo.20230024. eCollection 2024 Mar. Smart Mol. 2024. PMID: 40625522 Free PMC article.
-
Mechanisms and Clinical Implications of Human Gut Microbiota-Drug Interactions in the Precision Medicine Era.Biomedicines. 2024 Jan 16;12(1):194. doi: 10.3390/biomedicines12010194. Biomedicines. 2024. PMID: 38255298 Free PMC article. Review.
References
-
- Bhosle D. Bharambe S. Gairola N. Dhaneshwar S. S. Indian J. Pharm. Sci. 2006;68:286–294. doi: 10.4103/0250-474X.26654. - DOI
Publication types
LinkOut - more resources
Full Text Sources

