A new synthetic route for the preparation of [Os3(CO)10(μ-OH)(μ-H)] and its reaction with bis(diphenylphosphino)methane (dppm): syntheses and X-ray structures of two isomers of [Os3(CO)8(μ-OH)(μ-H)(μ-dppm)] and [Os3(CO)7(μ3-CO)(μ3-O)(μ-dppm)]
- PMID: 35516232
- PMCID: PMC9058515
- DOI: 10.1039/d0ra08783a
A new synthetic route for the preparation of [Os3(CO)10(μ-OH)(μ-H)] and its reaction with bis(diphenylphosphino)methane (dppm): syntheses and X-ray structures of two isomers of [Os3(CO)8(μ-OH)(μ-H)(μ-dppm)] and [Os3(CO)7(μ3-CO)(μ3-O)(μ-dppm)]
Abstract
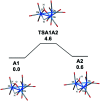
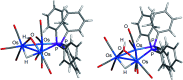
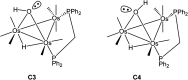
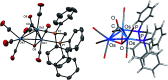
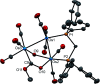
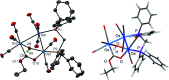
The triosmium cluster [Os3(CO)10(μ-OH)(μ-H)] containing bridging hydride and hydroxyl groups at a common Os-Os edge was obtained in good yield (ca. 75%) from the hydrolysis of the labile triosmium cluster [Os3(CO)10(NCMe)2] in THF at 67 °C. [Os3(CO)10(μ-OH)(μ-H)] reacts with dppm at 68 °C to afford the isomeric clusters 1 and 2 with the general formula [Os3(CO)8(μ-OH)(μ-H)(μ-dppm)] that differ by the disposition of bridging dppm ligand. Cluster 1 is produced exclusively from the reaction of [Os3(CO)10(μ-OH)(μ-H)] with dppm in CH2Cl2 at room temperature in the presence of added Me3NO. Heating cluster 1 at 81 °C furnishes 2 in a process that likely proceeds by the release of one arm of the dppm ligand, followed by ligand reorganization about the cluster polyhedron and ring closure of the pendent dppm ligand. The oxo-capped [Os3(CO)7(μ3-CO)(μ3-O)(μ-dppm)] (3) has been isolated starting from the thermolysis of either 1 or 2 at 139 °C. Reactions of [Os3(CO)10(μ-dppm)] with ROH (R = Me, Et) in the presence of Me3NO at 80 °C furnish [Os3(CO)8(μ-OH)(μ,η1,κ1-OCOR)(μ-dppm)] (4, R = Me; 5, R = Et). Clusters 1-5 have been characterized by a combination of analytical and spectroscopic studies, and the molecular structure of each product has been established by X-ray crystallography. The bonding in these products has been examined by electronic structure calculations, and cluster 1 is confirmed as the kinetic product of substitution, while cluster 2 represents the thermodynamically favored isomer.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
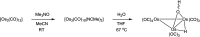
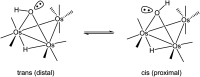
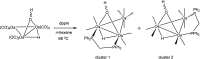


Similar articles
-
Reactions of diphosphine-stabilized Os3 clusters with triphenylantimony: syntheses and structures of new antimony-containing Os3 clusters via Sb-Ph bond cleavage.RSC Adv. 2023 Jan 18;13(5):2841-2851. doi: 10.1039/d2ra07284j. eCollection 2023 Jan 18. RSC Adv. 2023. PMID: 36756440 Free PMC article.
-
Opening of Carborane Cages by Metal Cluster Complexes: The Reaction of a Thiolate-Substituted Carborane with Triosmium Carbonyl Cluster Complexes.Inorg Chem. 2016 Aug 15;55(16):8207-13. doi: 10.1021/acs.inorgchem.6b01403. Epub 2016 Aug 3. Inorg Chem. 2016. PMID: 27487332
-
Reactivity of triruthenium thiophyne and furyne clusters: competitive S-C and P-C bond cleavage reactions and the generation of highly unsymmetrical alkyne ligands.Dalton Trans. 2008 Nov 28;(44):6219-30. doi: 10.1039/b806846a. Epub 2008 Sep 30. Dalton Trans. 2008. PMID: 18985255
-
Multiple cluster CH activations and transformations of furan by triosmium carbonyl complexes.Chem Commun (Camb). 2018 Apr 3;54(28):3464-3467. doi: 10.1039/C8CC01532E. Chem Commun (Camb). 2018. PMID: 29561037
-
Cage Opening of a Carborane Ligand by Metal Cluster Complexes.Chemistry. 2016 May 4;22(19):6501-4. doi: 10.1002/chem.201601075. Epub 2016 Mar 23. Chemistry. 2016. PMID: 26971388
Cited by
-
A Remarkably Unsymmetric Hexairon Core Embraced by Two High-Symmetry Tripodal Oligo-α-pyridylamido Ligands.Inorg Chem. 2023 Jul 3;62(26):10171-10184. doi: 10.1021/acs.inorgchem.3c00808. Epub 2023 Jun 21. Inorg Chem. 2023. PMID: 37345231 Free PMC article.
-
2-D Molecular Alloy Ru-M (M = Cu, Ag, and Au) Carbonyl Clusters: Synthesis, Molecular Structure, Catalysis, and Computational Studies.Inorg Chem. 2022 Sep 19;61(37):14726-14741. doi: 10.1021/acs.inorgchem.2c02099. Epub 2022 Sep 7. Inorg Chem. 2022. PMID: 36069711 Free PMC article.
References
-
- Johnson B. F. G. Lewis J. Kilty P. A. J. Chem. Soc. A. 1968:2859.
-
- Arce A. J. Deeming A. J. Donovan-Mtunzi S. Kabir S. E. J. Chem. Soc., Dalton Trans. 1985:2479.
-
- Banford J. Mays M. J. Raithby P. A. J. Chem. Soc., Dalton Trans. 1985:1355.
-
- Bryan E. G. Johnson B. F. G. Lewis J. J. Chem. Soc., Dalton Trans. 1977:1328.
-
- Roberto D. Lucenti E. Roveda C. Ugo R. Organometallics. 1997;16:5974.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

