The importance of Asn52 in the structure-function relationship of human cytochrome c
- PMID: 35516242
- PMCID: PMC9058552
- DOI: 10.1039/d0ra09961a
The importance of Asn52 in the structure-function relationship of human cytochrome c
Abstract
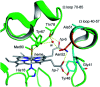
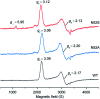
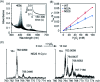
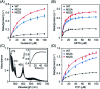
The function of the highly conserved residue Asn52 in human cytochrome c (H-Cyt c) is not fully understood. Herein, we show that the naturally occurring variant N52S H-Cyt c has a perturbed secondary structure, with a small fraction of high-spin species. Remarkably, it exhibits an enhanced peroxidase activity by 3-8-fold at neutral pH, as well as self-oxidation in reaction with H2O2. This study suggests that the H-bond network mediated by Asn52 is essential to suppress the apoptotic activity of H-Cyt c under physiological conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
The oxidative nuclease activity of human cytochrome c with mutations in Ω-loop C/D.Biochim Biophys Acta Proteins Proteom. 2023 May 1;1871(3):140897. doi: 10.1016/j.bbapap.2023.140897. Epub 2023 Jan 13. Biochim Biophys Acta Proteins Proteom. 2023. PMID: 36642204
-
A Compact Structure of Cytochrome c Trapped in a Lysine-Ligated State: Loop Refolding and Functional Implications of a Conformational Switch.J Am Chem Soc. 2015 Jul 8;137(26):8435-49. doi: 10.1021/jacs.5b01493. Epub 2015 Jun 24. J Am Chem Soc. 2015. PMID: 26038984 Free PMC article.
-
Effects of naturally occurring S47F/A mutations on the structure and function of human cytochrome c.J Inorg Biochem. 2023 Sep;246:112296. doi: 10.1016/j.jinorgbio.2023.112296. Epub 2023 Jun 17. J Inorg Biochem. 2023. PMID: 37356378
-
Apoptotic interactions of cytochrome c: redox flirting with anionic phospholipids within and outside of mitochondria.Biochim Biophys Acta. 2006 May-Jun;1757(5-6):648-59. doi: 10.1016/j.bbabio.2006.03.002. Epub 2006 Mar 31. Biochim Biophys Acta. 2006. PMID: 16740248 Review.
-
Multifunctional Cytochrome c: Learning New Tricks from an Old Dog.Chem Rev. 2017 Nov 8;117(21):13382-13460. doi: 10.1021/acs.chemrev.7b00257. Epub 2017 Oct 13. Chem Rev. 2017. PMID: 29027792 Review.
Cited by
-
Structural Dynamics of the Precatalytic State of Human Cytochrome c upon T28C, G34C, and A50C Mutations: A Molecular Dynamics Simulation Perspective.ACS Omega. 2023 Apr 17;8(17):15229-15238. doi: 10.1021/acsomega.3c00220. eCollection 2023 May 2. ACS Omega. 2023. PMID: 37151554 Free PMC article.
-
Naturally Occurring I81N Mutation in Human Cytochrome c Regulates Both Inherent Peroxidase Activity and Interactions with Neuroglobin.ACS Omega. 2022 Mar 22;7(13):11510-11518. doi: 10.1021/acsomega.2c01256. eCollection 2022 Apr 5. ACS Omega. 2022. PMID: 35415373 Free PMC article.
-
Altered conformational dynamics contribute to species-specific effects of cytochrome c mutations on caspase activation.J Biol Inorg Chem. 2024 Mar;29(2):169-176. doi: 10.1007/s00775-024-02044-2. Epub 2024 Mar 12. J Biol Inorg Chem. 2024. PMID: 38472487 Free PMC article.
-
Regulating Effect of Cytochrome b5 Overexpression on Human Breast Cancer Cells.Molecules. 2022 Jul 17;27(14):4556. doi: 10.3390/molecules27144556. Molecules. 2022. PMID: 35889429 Free PMC article.
References
-
- Bertini I. Cavallaro G. Rosato A. Chem. Rev. 2006;106:90–115. - PubMed
-
- Smith L. J. Kahraman A. Thornton J. M. Proteins. 2010;78:2349–2368. - PubMed
-
- Alvarez-Paggi D. Hannibal L. Castro M. A. Oviedo-Rouco S. Demicheli V. Tortora V. Tomasina F. Radi R. Murgida D. H. Chem. Rev. 2017;117:13382–13460. - PubMed
-
- Yoshikawa S. Shimada A. Chem. Rev. 2015;115:1936–1989. - PubMed
LinkOut - more resources
Full Text Sources

