Sustainable separation of bio-based cadaverine based on carbon dioxide capture by forming carbamate
- PMID: 35516266
- PMCID: PMC9058519
- DOI: 10.1039/d0ra08564b
Sustainable separation of bio-based cadaverine based on carbon dioxide capture by forming carbamate
Abstract
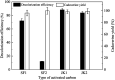
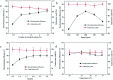
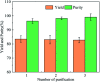
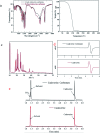
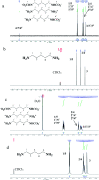
Bio-based cadaverine, manufactured by the decarboxylation of l-lysine, is an important raw material. However, the extractive-distillation separation and purification of cadaverine from bioconversion fluids require high energy consumption and leads to the loss of self-released carbon dioxide during the decarboxylation of l-lysine. This study focuses on the green and sustainable separation of bio-based cadaverine based on the capture of self-released carbon dioxide by cadaverine forming carbamate. Results showed that granular-activated carbon JK1 shows the best decolorization efficiency and achieves a higher cadaverine yield. After three times of solventing-out crystallization, refined cadaverine carbamate with 99.1% purity and total 57.48% yield was obtained. It was also found that the refined cadaverine carbamate consists of mixed crystals having numerous structural forms that can easily dissociate carbon dioxide. Furthermore, the amine carbamate strategy may be of great value for the development of a green and sustainable separation mode of bio-based amines and carbon dioxide capture.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
High-Level Conversion of l-lysine into Cadaverine by Escherichia coli Whole Cell Biocatalyst Expressing Hafnia alvei l-lysine Decarboxylase.Polymers (Basel). 2019 Jul 14;11(7):1184. doi: 10.3390/polym11071184. Polymers (Basel). 2019. PMID: 31337154 Free PMC article.
-
Ameliorating end-product inhibition to improve cadaverine production in engineered Escherichia coli and its application in the synthesis of bio-based diisocyanates.Synth Syst Biotechnol. 2021 Sep 14;6(4):243-253. doi: 10.1016/j.synbio.2021.09.004. eCollection 2021 Dec. Synth Syst Biotechnol. 2021. PMID: 34584992 Free PMC article.
-
Coupling the fermentation and membrane separation process for polyamides monomer cadaverine production from feedstock lysine.Eng Life Sci. 2021 Jun 10;21(10):623-629. doi: 10.1002/elsc.202000099. eCollection 2021 Oct. Eng Life Sci. 2021. PMID: 34690633 Free PMC article.
-
Green chemical and biological synthesis of cadaverine: recent development and challenges.RSC Adv. 2021 Jul 7;11(39):23922-23942. doi: 10.1039/d1ra02764f. eCollection 2021 Jul 6. RSC Adv. 2021. PMID: 35479032 Free PMC article. Review.
-
The cad locus of Enterobacteriaceae: more than just lysine decarboxylation.Anaerobe. 2009 Feb-Apr;15(1-2):1-6. doi: 10.1016/j.anaerobe.2008.05.002. Epub 2008 May 17. Anaerobe. 2009. PMID: 18572426 Review.
References
-
- Ma W. Chen K. Li Y. Hao N. Wang X. Ouyang P. Engineering. 2017;3:308–317.
-
- Chae T. U. Ahn J. H. Ko Y. Kim J. W. Lee J. A. Lee E. H. Lee S. Y. Metall. Eng. 2020;58:2–16. - PubMed
-
- Leong Y. K. Chen C. Huang S. Lin H. Li S. Ng I. Chang J. Biochem. Eng. J. 2020;157:107547.
-
- Matsuura R. Kishida M. Konishi R. Hirata Y. Adachi N. Segawa S. Imao K. Tanaka T. Kondo A. Biotechnol. Bioeng. 2019;116:2640–2651. - PubMed
LinkOut - more resources
Full Text Sources

