Luminescent zinc(ii) selone macrocyclic ring
- PMID: 35516307
- PMCID: PMC9064214
- DOI: 10.1039/c9ra01819k
Luminescent zinc(ii) selone macrocyclic ring
Abstract
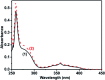
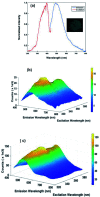
The synthesis and photophysical properties of macrocyclic Zn(ii) selone molecule have been reported. The structural property of Zn(ii) selone was elucidated using single crystal X-ray diffraction study. The solid-state structure of zinc(ii) selone molecule exhibits a perfect zinc(ii) selone 28 membered ring system with tetra coordination geometry around zinc(ii) center. The zinc(ii) selone ring system can be considered as the largest zinc(ii) ring system known without any non-interacting centered guest moiety. Detailed trends in photophysical as well as thermal properties were probed. In photoluminescence study, the solid-state sample of zinc(ii) selone ring system emits the bluish-yellow color with considerable quantum yields, while the solution state sample of zinc(ii) selone ring system in DMSO emits bluish-yellow. The luminescence lifetime of zinc(ii) selone was measured using standard time-correlated single photon counting (TCSPC) technique.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

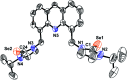






Similar articles
-
Fluorescent zinc(II) thione and selone complexes for light-emitting applications.Dalton Trans. 2025 Jan 21;54(4):1384-1392. doi: 10.1039/d4dt02924k. Dalton Trans. 2025. PMID: 39718013
-
Facile access to zinc and cadmium selones: highly active catalysts for Barbier reactions in aqueous media.Dalton Trans. 2016 Apr 21;45(15):6456-65. doi: 10.1039/c5dt04871k. Dalton Trans. 2016. PMID: 26952774
-
Study on the Photoluminescent and Thermal Properties of Zinc Complexes with a N₆O₄ Macrocyclic Ligand.Molecules. 2018 Jul 16;23(7):1735. doi: 10.3390/molecules23071735. Molecules. 2018. PMID: 30012984 Free PMC article.
-
Synthesis, characterization, DFT calculations, and electrochemical comparison of novel iron(ii) complexes with thione and selone ligands.Dalton Trans. 2016 Mar 21;45(11):4697-711. doi: 10.1039/c5dt03384e. Epub 2016 Feb 9. Dalton Trans. 2016. PMID: 26859480
-
[Supramolecular chemistry of multinuclear zinc(II) complexes in aqueous solution].Yakugaku Zasshi. 2002 Dec;122(12):1095-108. doi: 10.1248/yakushi.122.1095. Yakugaku Zasshi. 2002. PMID: 12510387 Review. Japanese.
Cited by
-
Catalytically active coordination polymer with a tiny Zn2Se2 ring bridged by bis-selone.RSC Adv. 2020 Aug 4;10(48):28950-28957. doi: 10.1039/d0ra04577b. eCollection 2020 Aug 3. RSC Adv. 2020. PMID: 35520066 Free PMC article.
References
-
- Zhang L. M. Li H. Y. Li H. X. Young D. J. Wang Y. Lang J. P. Inorg. Chem. 2017;56:11230–11243. doi: 10.1021/acs.inorgchem.7b01616. - DOI - PubMed
- Ghavale N. Manjare S. T. Singh H. B. Butcher R. J. Dalton Trans. 2015;44:11893–11900. doi: 10.1039/C5DT01565K. - DOI - PubMed
- Jin J. Shin H. W. Park J. H. Park J. H. Kim E. Ahn T. K. Ryu D. H. Son S. U. Organometallics. 2013;32:3954–3959. doi: 10.1021/om4004412. - DOI
- Alvarado E. Badaj A. C. Larocque T. G. Lavoie G. G. Chem.–Eur. J. 2012;18:12112–12121. doi: 10.1002/chem.201201448. - DOI - PubMed
- Srinivas K. Prabusankar G. Dalton Trans. 2017;46:16615–16622. doi: 10.1039/C7DT03796A. - DOI - PubMed
- Kim H. R. Jung I. G. Yoo K. Jang K. Lee E. S. Yun J. Son S. U. Chem. Commun. 2010;46:758–760. doi: 10.1039/B919515G. - DOI - PubMed
- Srinivas K. Babu C. N. Prabusankar G. Dalton Trans. 2015;44:15636–15644. doi: 10.1039/C5DT02320C. - DOI - PubMed
- Srinivas K. Prabusankar G. RSC Adv. 2018;8:32269–32282. doi: 10.1039/C8RA06057F. - DOI - PMC - PubMed
-
- Srinivas K. Sathyanarayana A. Babu C. N. Prabusankar G. Dalton Trans. 2016;45:5196–5209. doi: 10.1039/C5DT04738B. - DOI - PubMed
- Zhang H.-N. Jia W.-G. Xu Q.-T. Ji C.-C. Inorg. Chim. Acta. 2016;450:315–320. doi: 10.1016/j.ica.2016.06.023. - DOI
- Sharma A. K. Joshi H. Sharma K. N. Gupta P. L. Singh A. K. Organometallics. 2014;33:3629–3639. doi: 10.1021/om500579r. - DOI
- Huang Y. B. Jia W. G. Jin G. X. J. Organomet. Chem. 2009;694:86–90. doi: 10.1016/j.jorganchem.2008.10.009. - DOI
- Prabusankar G. Mannem A. Muthukumaran N. J. Organomet. Chem. 2019;884:29–35. doi: 10.1016/j.jorganchem.2019.01.017. - DOI
- Sharma A. K. Joshi H. Bhaskar R. Singh A. K. Dalton Trans. 2017;46:2228–2237. doi: 10.1039/C6DT04271F. - DOI - PubMed
-
- Williams D. J. White K. M. VanDerveer D. Wilkinson A. P. Inorg. Chem. Commun. 2002;5:124–126. doi: 10.1016/S1387-7003(01)00360-4. - DOI
LinkOut - more resources
Full Text Sources

