Catalyst-free four-component domino synthetic approach toward versatile multicyclic spirooxindole pyran scaffolds
- PMID: 35516360
- PMCID: PMC9064459
- DOI: 10.1039/c9ra03214b
Catalyst-free four-component domino synthetic approach toward versatile multicyclic spirooxindole pyran scaffolds
Abstract
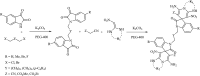
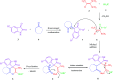
A new versatile strategy involving a sequential four-component reaction of the nitroketene dithioacetals, alkylamine/benzylamine, isatin and various enolizable active methylene structures (pyrazolone, barbituric acid, 1,3-indandione and 2-hydroxy-1,4-naphthoquinone) as precursors under mild and catalyst-free conditions results in the synthesis of new functionalized spirooxindole pyrans named spiro[indoline-3,4'-pyrano[2,3-c]pyrazol], spiro[indoline-3,5'-pyrano[2,3-d]pyrimidine], spiro[indeno[1,2-b]pyran-4,3'-indoline] and spiro[benzo[g]chromene-4,3'-indoline] in moderate to good yields. The use of various active methylene compounds affords a range of skeletally distinct spirooxindole-based heterocycles with potential biological properties. The present strategy has many advantages, such as convenient one-pot operation, simple workup procedures and straightforward isolation without using tedious purification steps such as column chromatography, progress under catalyst-free condition and high molecular diversity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
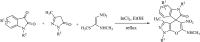

Similar articles
-
Sonochemistry in an organocatalytic domino reaction: an expedient multicomponent access to structurally functionalized dihydropyrano[3,2-b]pyrans, spiro-pyrano[3,2-b]pyrans, and spiro-indenoquinoxaline-pyranopyrans under ambient conditions.RSC Adv. 2022 Apr 28;12(20):12843-12857. doi: 10.1039/d2ra01917e. eCollection 2022 Apr 22. RSC Adv. 2022. PMID: 35496344 Free PMC article.
-
Synthesis of new functionalized thiazolo pyridine-fused and thiazolo pyridopyrimidine-fused spirooxindoles via one-pot reactions.Heliyon. 2020 Mar 31;6(3):e03687. doi: 10.1016/j.heliyon.2020.e03687. eCollection 2020 Mar. Heliyon. 2020. PMID: 32258502 Free PMC article.
-
Three-component synthesis of spiro[indoline-3,4'-pyrano[3,2-b]pyran]- 2,8'-diones using a one-pot reaction.Comb Chem High Throughput Screen. 2014 Feb;17(2):132-40. doi: 10.2174/13862073113166660067. Comb Chem High Throughput Screen. 2014. PMID: 24164049
-
Recent Strategies in the Synthesis of Spiroindole and Spirooxindole Scaffolds.Top Curr Chem (Cham). 2021 May 18;379(4):25. doi: 10.1007/s41061-021-00337-7. Top Curr Chem (Cham). 2021. PMID: 34002298 Review.
-
Update on thiopyran-fused heterocycle synthesis (2013-2024).Org Biomol Chem. 2024 Jul 17;22(28):5676-5717. doi: 10.1039/d4ob00497c. Org Biomol Chem. 2024. PMID: 38912843 Review.
Cited by
-
A one-pot synthesis of piperidinium spirooxindoline-pyridineolates and indole-substituted pyridones in aqueous or ethanol medium.Mol Divers. 2022 Aug;26(4):2039-2048. doi: 10.1007/s11030-021-10313-4. Epub 2021 Sep 15. Mol Divers. 2022. PMID: 34528212
-
Synthesis of diverse spiro-imidazo pyridine-indene derivatives via acid-promoted annulation reaction of bindone and heterocyclic ketene aminals.Sci Rep. 2022 Jul 22;12(1):12550. doi: 10.1038/s41598-022-16959-w. Sci Rep. 2022. PMID: 35869174 Free PMC article.
References
-
- Gajulapalli V. P. R. Lokesh K. Vishwanath M. Kesavan V. RSC Adv. 2016;6:12180–12184. doi: 10.1039/C5RA25025K. - DOI
-
- Us D. Gürdal E. Berk B. Öktem S. Kocagöz T. Çağlayan B. Erol D. D. Turk. J. Chem. 2009;33:803–812.
LinkOut - more resources
Full Text Sources

