Solvent-driven structural topology involving energetically significant intra- and intermolecular chelate ring contacts and anticancer activities of Cu(ii) phenanthroline complexes involving benzoates: experimental and theoretical studies
- PMID: 35516385
- PMCID: PMC9064362
- DOI: 10.1039/c9ra01181a
Solvent-driven structural topology involving energetically significant intra- and intermolecular chelate ring contacts and anticancer activities of Cu(ii) phenanthroline complexes involving benzoates: experimental and theoretical studies
Abstract
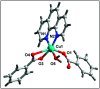
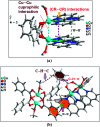
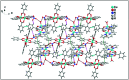
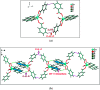
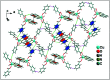
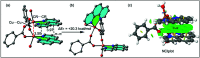
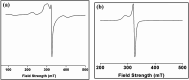
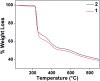
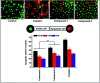
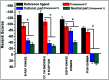
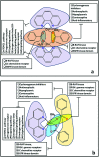
Two new coordination solids [Cu2(μ2-Bz)4(CH3OH)2][Cu2(η2-Bz)2(phen)2(H2O)2]·(NO3)2 (1) and [Cu(phen)(H2O)(Bz)(η2-Bz)] (2) (phen = 1,10-phenanthroline; Bz = benzoate) have been synthesized and characterized using elemental analysis, TGA, spectroscopic (IR, UV-vis-NIR and ESR) and single crystal X-ray diffraction techniques. Change of the solvent from methanol to DMF results in changes in the architectures that are triggered by a change from square pyramidal to octahedral coordination at the divalent metal centers for complexes 1 and 2 respectively. The structural topology of the complexes is established by the interplay of strong O-H⋯O and weak C-H⋯O, C-H⋯C, π-π stacking interactions. Unconventional parallel intramolecular and anti-parallel intermolecular contacts involving the chelate rings (CR) also stabilize the structures. The energetic analyses of the structures evidence that the parallel arrangement is energetically favoured which is likely due to the presence of the Cu⋯Cu cuprophilic interaction in 1 that is not established in 2. Compound 1 exhibits the highest antibacterial activity against Rhizobium leguminosarum among the tested cultures. In vitro cytotoxicity and apoptosis studies were carried out for compounds 1 and 2 on malignant Dalton's lymphoma cell line (DL). Both compounds showed a significant effect on the decrease in cell viability as compared to a control, while compound 2 induced remarkable cytotoxicity towards DL cells. Treatment also showed the appearance of membrane blebbing, chromatin condensation and fragmented nuclei which are typical characteristic features of apoptotic cell death. Furthermore, a docking study revealed that both compounds docked in the active sites of all the cancer target proteins under study. Moreover, SAR analysis revealed that oxygen and nitrogen atoms of compound 1 and the oxygen atoms of compound 2 are crucial for biological activities.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









Similar articles
-
Adipato bridged novel hexanuclear Cu(ii) and polymeric Co(ii) coordination compounds involving cooperative supramolecular assemblies and encapsulated guest water clusters in a square grid host: antiproliferative evaluation and theoretical studies.Dalton Trans. 2020 Jul 21;49(28):9863-9881. doi: 10.1039/d0dt01007c. Dalton Trans. 2020. PMID: 32638786
-
Effects of N-oxidation on the molecular and crystal structures and properties of isocinchomeronic acid, its metal complexes and their supramolecular architectures: experimental, CSD survey, solution and theoretical approaches.RSC Adv. 2019 Aug 14;9(44):25382-25404. doi: 10.1039/c9ra05143k. eCollection 2019 Aug 13. RSC Adv. 2019. PMID: 35530069 Free PMC article.
-
Polyoxometalate-supported transition metal complexes and their charge complementarity: synthesis and characterization of [M(OH)6Mo6O18[Cu(Phen)(H2O)2]2][M(OH)6Mo6O18[Cu(Phen)(H2O)Cl]2].5H2O (M = Al(+, Cr3+).Inorg Chem. 2005 Nov 28;44(24):8846-54. doi: 10.1021/ic050830j. Inorg Chem. 2005. PMID: 16296839
-
Solvent effects on the crystallization and structure of ternary copper(ii) coordination compounds with l-threonine and 1,10-phenanthroline.Heliyon. 2022 Jun 3;8(6):e09556. doi: 10.1016/j.heliyon.2022.e09556. eCollection 2022 Jun. Heliyon. 2022. PMID: 35694423 Free PMC article.
-
Solvatomorphic Diversity in Coordination Compounds of Copper(II) with l-Homoserine and 1,10-Phenanthroline: Syntheses, Crystal Structures and ESR Study.Molecules. 2024 Nov 27;29(23):5621. doi: 10.3390/molecules29235621. Molecules. 2024. PMID: 39683780 Free PMC article.
Cited by
-
Osmium Arene Germyl, Stannyl, Germanate, and Stannate Complexes as Anticancer Agents.ACS Omega. 2021 Jul 12;6(29):19252-19268. doi: 10.1021/acsomega.1c02665. eCollection 2021 Jul 27. ACS Omega. 2021. PMID: 34337263 Free PMC article.
References
-
- Cahill C. L. De Lill D. T. Frisch M. CrystEngComm. 2007;9:15–26. doi: 10.1039/B615696G. - DOI
-
- Maspoch D. Ruiz-Molina D. Veciana J. Chem. Soc. Rev. 2007;36:770–818. doi: 10.1039/B501600M. - DOI - PubMed
- Robin A. Y. Fromm K. M. Coord. Chem. Rev. 2006;250:2127–2157. doi: 10.1016/j.ccr.2006.02.013. - DOI
- Carlucci L. Ciani G. Proserpio D. M. Coord. Chem. Rev. 2003;246:247–289. doi: 10.1016/S0010-8545(03)00126-7. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

