Alzheimer's disease drug development pipeline: 2022
- PMID: 35516416
- PMCID: PMC9066743
- DOI: 10.1002/trc2.12295
Alzheimer's disease drug development pipeline: 2022
Abstract
Introduction: Alzheimer's disease (AD) represents a global health crisis. Treatments are needed to prevent, delay the onset, slow the progression, improve cognition, and reduce behavioral disturbances of AD. We review the current clinical trials and drugs in development for the treatment of AD.
Methods: We searched the governmental website clinicaltrials.gov where are all clinical trials conducted in the United States must be registered. We used artificial intelligence (AI) and machine learning (ML) approaches to ensure comprehensive detection and characterization of trials and drugs in development. We use the Common Alzheimer's Disease Research Ontology (CADRO) to classify drug targets and mechanisms of action of drugs in the pipeline.
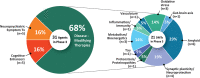
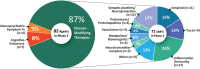
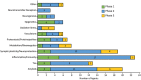
Results: As of January 25, 2022 (index date for this study) there were 143 agents in 172 clinical trials for AD. The pipeline included 31 agents in 47 trials in Phase 3, 82 agents in 94 trials in Phase 2, and 30 agents in 31 trials in Phase 1. Disease-modifying therapies represent 83.2% of the total number of agents in trials; symptomatic cognitive enhancing treatments represent 9.8% of agents in trials; and drugs for the treatment of neuropsychiatric symptoms comprise 6.9%. There is a diverse array of drug targets represented by agents in trials including nearly all CADRO categories. Thirty-seven percent of the candidate agents in the pipeline are repurposed drugs approved for other indications. A total of 50,575 participants are needed to fulfill recruitment requirements for all currently active clinical trials.
Discussion: The AD drug development pipeline has agents representing a substantial array of treatment mechanisms and targets. Advances in drug design, outcome measures, use of biomarkers, and trial conduct promise to accelerate the delivery of new and better treatments for patients with AD.
Highlights: There are 143 drugs in the current Alzheimer's disease (AD) drug development pipeline.Disease-modifying therapies represent 83.2% of the candidate treatments.Current trials require 50,575 participants who will donate 3,878,843 participant-weeks to clinical trials.The biopharmaceutical industry sponsors 50% of all clinical trials including 68% of Phase 3 trials.Sixty-three percent of Phase 3 trials and 46% of Phase 2 trials include non-North American clinical trial site locations indicating the global ecosystem required for AD drug development.
Keywords: Alzheimer's disease; Common Alzheimer's Disease Research Ontology (CADRO); aducanumab; amyloid; biomarkers; clinical trials; donanemab; drug development; inflammation; lecanemab; pharmaceutical companies; repurposed drugs; tau.
© 2022 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Jeffrey Cummings has provided consultation to Acadia, Alkahest, AlphaCognition, AriBio, Avanir, Axsome, Behren Therapeutics, Biogen, Biohaven, Cassava, Cerecin, Cortexyme, Diadem, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Otsuka, PRODEO, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, United Neuroscience, and Unlearn AI pharmaceutical, assessment, and investment companies. Jeffrey Cummings owns the copyright of the Neuropsychiatric Inventory. Garam Lee is a full‐time employee of Biogen. Kate Zhong provides consultation to Green Valley Pharmaceuticals. Pouyan Nahed has no disclosures. Mina Esmail Zadeh Nojoo Kambar has no disclosures. Jorge Fonseca has no disclosures. Kate Zhong has no disclosures.
Figures

References
-
- Alzheimer's Association . 2021 Alzheimer's disease facts and figures. Alzheimer's Dement. 2021;17:327‐406. - PubMed
-
- Rabinovici GD. Controversy and progress in Alzheimer's disease—FDA approval of aducanumab. N Engl J Med. 2021;385:771‐774. - PubMed
-
- Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384:1691‐1704. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
