Oral Shedding of an Oncogenic Virus Alters the Oral Microbiome in HIV+ Patients
- PMID: 35516440
- PMCID: PMC9063630
- DOI: 10.3389/fmicb.2022.882520
Oral Shedding of an Oncogenic Virus Alters the Oral Microbiome in HIV+ Patients
Abstract
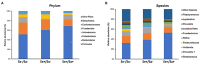
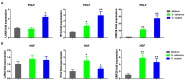
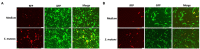
Kaposi's Sarcoma (KS) caused by Kaposi's sarcoma-associated herpesvirus (KSHV) continues to be the most common AIDS-associated tumor. Involvement of the oral cavity represents one of the most common clinical manifestations of this tumor. Numerous types of cancer are associated with the alterations of in components of the microbiome. However, little is known about how KSHV coinfection affects the oral microbiome in HIV+ patients, especially in a "pre-cancer" niche. Using 16S rRNA pyrosequencing, we found that oral shedding of KSHV correlated with altered oral microbiome signatures in HIV+ patients, including a reduction in the microbiota diversity, changing the relative composition of specific phyla and species, and regulating microbial functions. Furthermore, we found that Streptococcus sp., one of the most increased species in the oral cavity of HIV+/KSHV+ patients, induced KSHV lytic reactivation in primary oral cells. Together, these data indicate that oral shedding of KSHV may manipulate the oral microbiome to promote viral pathogenesis and tumorigenesis especially in immunocompromised patients.
Keywords: HIV; KSHV; microbiome; oncogenic virus; oral microbiota.
Copyright © 2022 Dai, Lu, Chen, Plaisance-Bonstaff, Mu, Forrest, Whitby, Post and Qin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Signatures of oral microbiome in HIV-infected individuals with oral Kaposi's sarcoma and cell-associated KSHV DNA.PLoS Pathog. 2020 Jan 17;16(1):e1008114. doi: 10.1371/journal.ppat.1008114. eCollection 2020 Jan. PLoS Pathog. 2020. PMID: 31951641 Free PMC article. Clinical Trial.
-
HIV-1 Tat Interacts with a Kaposi's Sarcoma-Associated Herpesvirus Reactivation-Upregulated Antiangiogenic Long Noncoding RNA, LINC00313, and Antagonizes Its Function.J Virol. 2020 Jan 17;94(3):e01280-19. doi: 10.1128/JVI.01280-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31723026 Free PMC article.
-
Porphyromonas gingivalis coinfects with KSHV in oral cavities of HIV+ patients and induces viral lytic reactivation.J Med Virol. 2020 Dec;92(12):3862-3867. doi: 10.1002/jmv.26028. Epub 2020 Jun 2. J Med Virol. 2020. PMID: 32436999 Free PMC article.
-
The Role of Bacteria in KSHV Infection and KSHV-Induced Cancers.Cancers (Basel). 2021 Aug 25;13(17):4269. doi: 10.3390/cancers13174269. Cancers (Basel). 2021. PMID: 34503079 Free PMC article. Review.
-
Kaposi's Sarcoma-Associated Herpesvirus: Epidemiology and Molecular Biology.Adv Exp Med Biol. 2017;1018:91-127. doi: 10.1007/978-981-10-5765-6_7. Adv Exp Med Biol. 2017. PMID: 29052134 Review.
Cited by
-
Human immunodeficiency virus and oral microbiota: mutual influence on the establishment of a viral gingival reservoir in individuals under antiretroviral therapy.Front Cell Infect Microbiol. 2024 Apr 10;14:1364002. doi: 10.3389/fcimb.2024.1364002. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38660490 Free PMC article. Review.
-
Role of the Microbiota in Skin Neoplasms: New Therapeutic Horizons.Microorganisms. 2023 Sep 25;11(10):2386. doi: 10.3390/microorganisms11102386. Microorganisms. 2023. PMID: 37894044 Free PMC article. Review.
References
-
- Benavente Y., Mbisa G., Labo N., Casabonne D., Becker N., Maynadie M., et al. . (2011). Antibodies against lytic and latent Kaposi’s sarcoma-associated herpes virus antigens and lymphoma in the European EpiLymph case-control study. Br. J. Cancer 105, 1768–1771. doi: 10.1038/bjc.2011.392, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

