Recent progress to construct calixarene-based polymers using covalent bonds: synthesis and applications
- PMID: 35516464
- PMCID: PMC9056661
- DOI: 10.1039/d0ra05707j
Recent progress to construct calixarene-based polymers using covalent bonds: synthesis and applications
Abstract
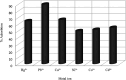
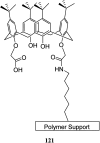
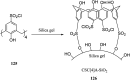
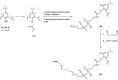
The combination of supramolecular chemistry and polymer sciences creates a great possibility to afford calixarene-based polymers offering unique features and applications. The enhancement of calixarene's versatility in this manner has made chemists better able to achieve different objectives in host-guest chemistry. The calixarene-based polymers can be divided into covalent polymers and supramolecular polymers regarding the interactions. Although there are several studies available on the calixarene-based supramolecular polymers, there is a paucity of studies on the calixarene-based covalent polymers. In this paper, the most recent developments and applications of the calixarene-based covalent polymers in the last two decades have been reviewed. We have particularly focused on the polymers, including those where the calixarene molecules have been used as macromonomers and polymerize through covalent bonds. Moreover, covalent polymers or solid supports functionalized with calixarenes are highlighted as well.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


























Similar articles
-
Calixarene-based supramolecular polymerization in solution.Chem Soc Rev. 2012 Sep 21;41(18):5907-21. doi: 10.1039/c2cs35075k. Epub 2012 May 22. Chem Soc Rev. 2012. PMID: 22617955
-
Calixarene Derivatives: A Mini-Review on their Synthesis and Demands in Nanosensors and Biomedical Fields.Mini Rev Med Chem. 2023;23(6):734-745. doi: 10.2174/1389557522666220928120727. Mini Rev Med Chem. 2023. PMID: 36173047 Review.
-
Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications.Acc Chem Res. 2014 Jul 15;47(7):1925-34. doi: 10.1021/ar500009g. Epub 2014 Mar 25. Acc Chem Res. 2014. PMID: 24666259 Review.
-
Engineering orthogonality in supramolecular polymers: from simple scaffolds to complex materials.Acc Chem Res. 2014 Aug 19;47(8):2405-16. doi: 10.1021/ar500128w. Epub 2014 Jun 6. Acc Chem Res. 2014. PMID: 24905869
-
Crown Ether-Based Supramolecular Polymers: From Synthesis to Self-Assembly.Macromol Rapid Commun. 2022 Jul;43(14):e2100775. doi: 10.1002/marc.202100775. Epub 2021 Dec 24. Macromol Rapid Commun. 2022. PMID: 34882882 Review.
Cited by
-
Advancing Adsorption and Separation with Modified SBA-15: A Comprehensive Review and Future Perspectives.Molecules. 2024 Jul 27;29(15):3543. doi: 10.3390/molecules29153543. Molecules. 2024. PMID: 39124948 Free PMC article. Review.
-
Synthesis of Ionizable Calix[4]arenes for Chelation of Selected Divalent Cations.Molecules. 2022 Feb 22;27(5):1478. doi: 10.3390/molecules27051478. Molecules. 2022. PMID: 35268577 Free PMC article.
-
Synthesis of a new chitosan-p-tert-butylcalix[4]arene polymer as adsorbent for toxic mercury ion.R Soc Open Sci. 2022 May 24;9(5):211223. doi: 10.1098/rsos.211223. eCollection 2022 May. R Soc Open Sci. 2022. PMID: 35620011 Free PMC article.
-
Synthesis and characterization of a new reusable calix[4]arene-bonded silica gel sorbent for antidiabetic drugs.RSC Adv. 2022 Sep 5;12(39):25123-25132. doi: 10.1039/d2ra04530c. eCollection 2022 Sep 5. RSC Adv. 2022. PMID: 36199337 Free PMC article.
References
-
- Gutsche C. D. Alam I. Iqbal M. Mangiafico T. Nam K. C. Rogers J. See K. A. Topics in calixarene chemistry. J. Inclusion Phenom. Mol. Recognit. Chem. 1989;7(1):61–72. doi: 10.1007/BF01112783. - DOI
-
- Gutsche C. D. Dhawan B. No K. H. Muthukrishnan R. Calixarenes. 4. The synthesis, characterization, and properties of the calixarenes from p-tert-butylphenol. J. Am. Chem. Soc. 1981;103(13):3782–3792. doi: 10.1021/ja00403a028. - DOI
-
- Balami U. Taylor D. K. Electrochemical responsive arrays of sulfonatocalixarene groups prepared by free radical polymerization. React. Funct. Polym. 2014;81:54–60. doi: 10.1016/j.reactfunctpolym.2014.03.015. - DOI
-
- Mendes A. R. Gregório C. C. Barata P. D. Costa A. I. Prata J. V. Linear and crosslinked copolymers of p-tert-butylcalix[4]arene derivatives and styrene: new synthetic approaches to polymer-bound calix[4]arenes. React. Funct. Polym. 2005;65(1–2):9–21. doi: 10.1016/j.reactfunctpolym.2005.01.006. - DOI
Publication types
LinkOut - more resources
Full Text Sources

