One-pot construction of carbohydrate scaffolds mediated by metal catalysts
- PMID: 35516477
- PMCID: PMC9056687
- DOI: 10.1039/d0ra05355d
One-pot construction of carbohydrate scaffolds mediated by metal catalysts
Abstract
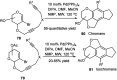
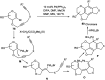
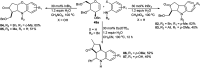
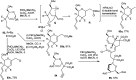
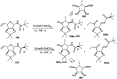
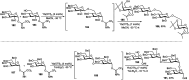
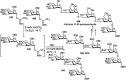
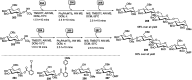
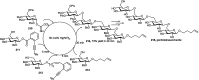
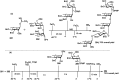
Owing to the environmental concern worldwide and also due to cost, time and labour issues, use of one-pot reactions [domino/cascade/tandem/multi-component (MC) or sequential] has gained much attention among the scientific and industrial communities for the generation of compound libraries having different scaffolds. Inclusion of sugars in such compounds is expected to increase the pharmacological efficacy because of the possibility of better interactions with the receptors of such unnatural glycoconjugates. In many of the one-pot transformations, the presence of a metal salt/complex can improve the reaction/change the course of reaction with remarkable increase in chemo-/regio-/stereo-selectivity. On the other hand because of the importance of natural polymeric glycoconjugates in life processes, the development and efficient synthesis of related oligosaccharides, particularly utilising one-pot MC-glycosylation techniques are necessary. The present review is an endeavour to discuss one-pot transformations involving carbohydrates catalysed by a metal salt/complex.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





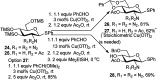

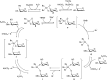
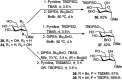
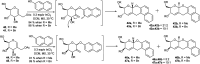






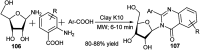
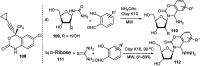
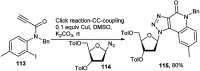







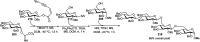




References
-
-
For reviews or books on Domino reactions:
- Tietze L. F. Chem. Rev. 1996;96:115–136. doi: 10.1021/cr950027e. - DOI - PubMed
- Pellissier H. Chem. Rev. 2013;113:442–524. doi: 10.1021/cr300271k. - DOI - PubMed
- Domino and intramolecular rearrangement reactions as advanced synthesis methods in glycosciences, ed. Z. J. Witczak and R. Bielski, John Wiley & Sons, 2016, p. 368
-
; For cascade reactions:
- Metal catalysed cascade reactions, in Topics in organometallic chemistry, ed. T. J. J. Muller, Springer, Berlin, Germany, 2006, vol. 19, p. 339
- Nicolaou K. C. Edmonds D. J. Bulger P. l. G. Angew. Chem., Int. Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. - DOI - PubMed
- Nicolaou K. C. Chen J. S. Chem. Soc. Rev. 2009;38:2993–3009. doi: 10.1039/B903290H. - DOI - PMC - PubMed
- Ohno H. Inuki S. Synthesis. 2018;50:700–710. doi: 10.1055/s-0036-1589165. - DOI
-
; For tandem reactions:
- Parsons P. J. Penkett C. S. Shell A. J. Chem. Rev. 1996;96:195–206. doi: 10.1021/cr950023+. - DOI - PubMed
- Wasilke J.-C. Obrey S. J. Baker R. T. Bazan G. C. Chem. Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n. - DOI - PubMed
- Chapman C. J. Frost C. G. Synthesis. 2007:1–21.
-
; For one-pot multicomponent reactions:
- Dömling A. Chem. Rev. 2006;106:17–89. doi: 10.1021/cr0505728. - DOI - PubMed
- Sunderhaus J. D. Martin S. F. Chemistry. 2009;15:1300–1308. doi: 10.1002/chem.200802140. - DOI - PMC - PubMed
- Brauch S. Van Berkel S. S. Westermann B. Chem. Soc. Rev. 2013;42:4948–4962. doi: 10.1039/C3CS35505E. - DOI - PubMed
- Koszytkowska-Stawińska M. Buchowicz W. Beilstein J. Org. Chem. 2014;10:1706–1732. doi: 10.3762/bjoc.10.179. - DOI - PMC - PubMed
- Rossi B. Pastori N. Prosperini S. Punta C. Beilstein J. Org. Chem. 2015;11:66–73. doi: 10.3762/bjoc.11.10. - DOI - PMC - PubMed
- Malinakova H. C. Rep. Org. Chem. 2015;5:75–90. doi: 10.2147/ROC.S65115. - DOI
- Khan Md. M. Yousuf R. Khan S. Shafiullah RSC Adv. 2015;5:57883–57905. doi: 10.1039/C5RA08059B. - DOI
- Khan Md. M. Khan S. Saigal Iqbal S. RSC Adv. 2016;6:42045–42061. doi: 10.1039/C6RA06767K. - DOI
-
-
- Jackman J. E. Fierke C. A. Tumey L. N. Pirrung M. Uchiyama T. Tahir S. H. Hindsgaul O. Raetz C. R. J. Biol. Chem. 2000;275:11002–11009. doi: 10.1074/jbc.275.15.11002. - DOI - PubMed
- Hang H. C. Bertozzi C. R. J. Am. Chem. Soc. 2001;123:1242–1243. doi: 10.1021/ja002962b. - DOI - PubMed
- Li X. Uchiyama T. Raetz C. R. Hindsgaul O. Org. Lett. 2003;5:539–541. doi: 10.1021/ol027458l. - DOI - PubMed
- Liu X. Guo Y. Li Y. Jiang Y. Chubb S. Azuma A. Huang P. Matsuda A. Hittelman W. Plunkett W. Cancer Res. 2005;65:6874–6881. doi: 10.1158/0008-5472.CAN-05-0288. - DOI - PubMed
- Velter I. La Ferla B. Nicotra F. J. Carbohydr. Chem. 2006;25:97–138. doi: 10.1080/07328300600733020. - DOI
- Bernardi A. Cheshev P. Chem.–Eur. J. 2008;14:7434–7441. doi: 10.1002/chem.200800597. - DOI - PubMed
- Lepenies B. Yin J. Seeberger P. H. Curr. Opin. Chem. Biol. 2010;14:404–411. doi: 10.1016/j.cbpa.2010.02.016. - DOI - PubMed
- Bouché L. Reissig H.-U. Pure Appl. Chem. 2012;84:23–36.
-
- Essentials of glycobiology, ed. A. Varki, R. D. Cummings, J. D. Esko, H. H. Freeze, P. Stanley, C. R. Bertozzi, G. W. Hart and M. E. Etzler, C.S. Harbor Press, NY, 2009 - PubMed
- Seeberger P. H. and Cummings R. D., Glycans in Biotechnology and Pharmaceutical Industry, in Essentials of Glycobiology, Executive ed. A. Varki, C.S. Harbor Press, NY, 3rd edn, 2017, ch. 57, p. 823
- Rudd P. M. Dwek R. A. Curr. Opin. Struct. Biol. 2006;16:559–560. doi: 10.1016/j.sbi.2006.09.002. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

