A decennary update on applications of metal nanoparticles (MNPs) in the synthesis of nitrogen- and oxygen-containing heterocyclic scaffolds
- PMID: 35516511
- PMCID: PMC9056690
- DOI: 10.1039/d0ra02272a
A decennary update on applications of metal nanoparticles (MNPs) in the synthesis of nitrogen- and oxygen-containing heterocyclic scaffolds
Abstract
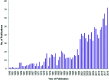
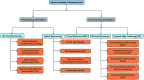
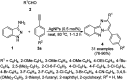
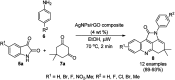
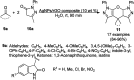
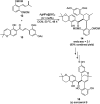
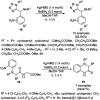
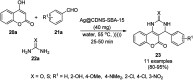
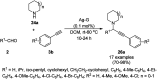
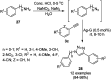
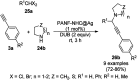
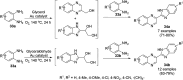
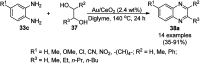
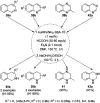
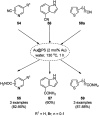
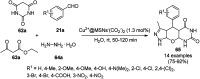
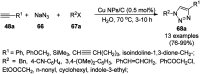
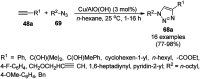
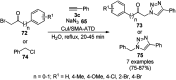
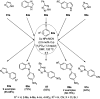
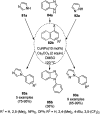
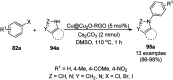
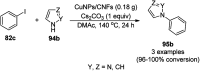
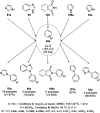
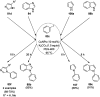
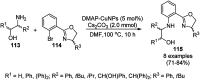
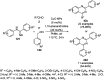
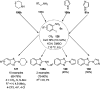
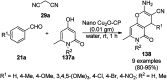
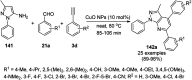
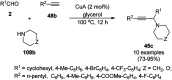
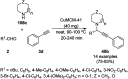
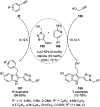
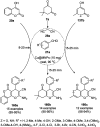
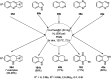
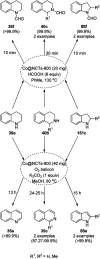
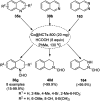
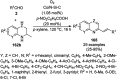
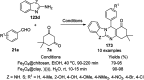
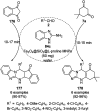
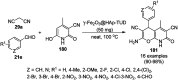
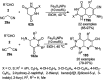
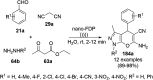
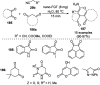
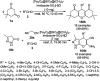
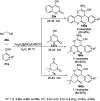
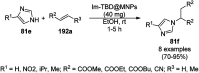
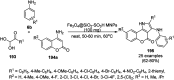
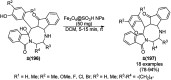
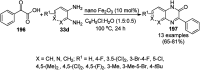
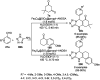
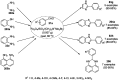
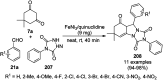
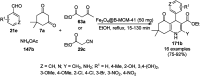
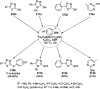
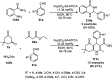
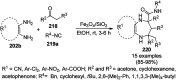
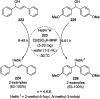
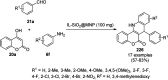
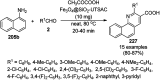
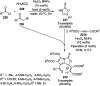
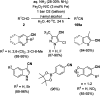
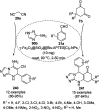
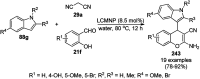
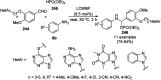
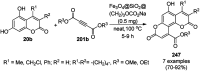
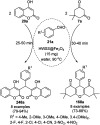
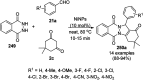
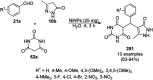
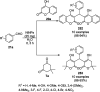
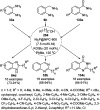
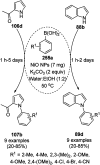
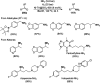
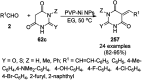
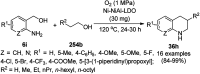
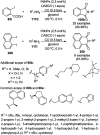
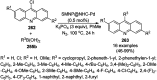
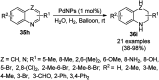
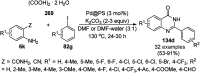
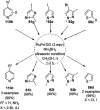
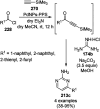
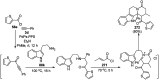
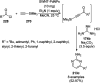
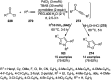
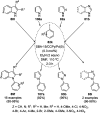
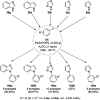
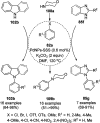
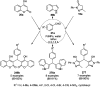
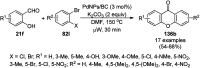
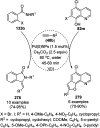
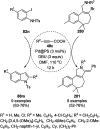
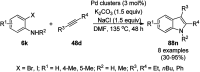
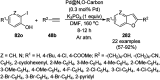
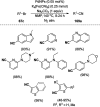
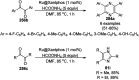
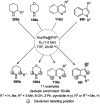
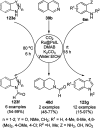
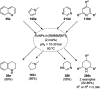
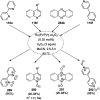
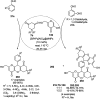
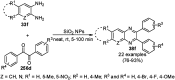
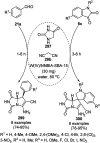
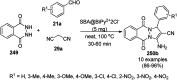
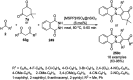
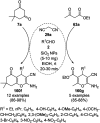
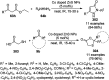
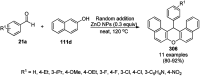
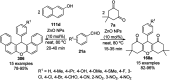
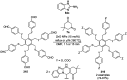
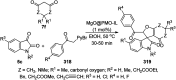
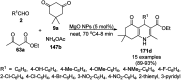
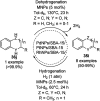
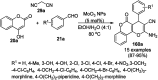
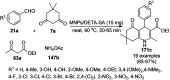
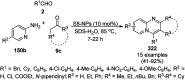
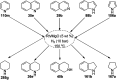
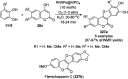
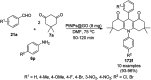
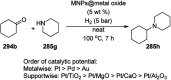
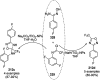
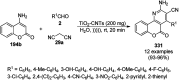
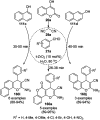
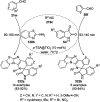
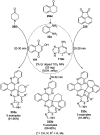
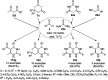
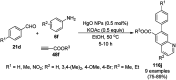
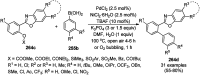
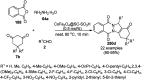
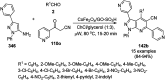
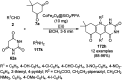
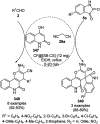
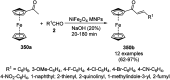
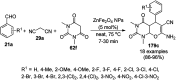
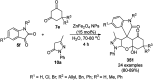
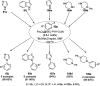
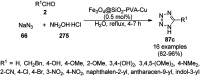
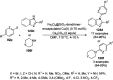
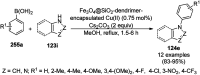
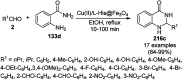
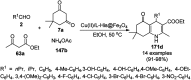
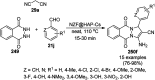
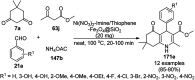
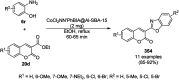
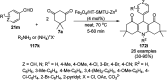
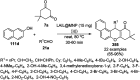
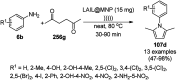
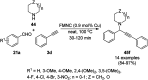
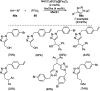
Heterocycles have been found to be of much importance as several nitrogen- and oxygen-containing heterocycle compounds exist amongst the various USFDA-approved drugs. Because of the advancement of nanotechnology, nanocatalysis has found abundant applications in the synthesis of heterocyclic compounds. Numerous nanoparticles (NPs) have been utilized for several organic transformations, which led us to make dedicated efforts for the complete coverage of applications of metal nanoparticles (MNPs) in the synthesis of heterocyclic scaffolds reported from 2010 to 2019. Our emphasize during the coverage of catalyzed reactions of the various MNPs such as Ag, Au, Co, Cu, Fe, Ni, Pd, Pt, Rh, Ru, Si, Ti, and Zn has not only been on nanoparticles catalyzed synthetic transformations for the synthesis of heterocyclic scaffolds, but also provide an inherent framework for the reader to select a suitable catalytic system of interest for the synthesis of desired heterocyclic scaffold.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



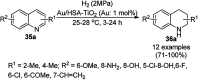
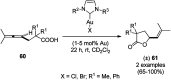
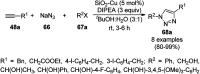

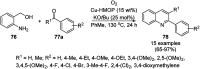


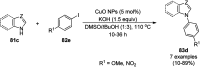
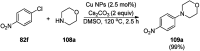
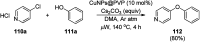




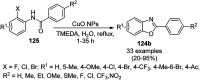
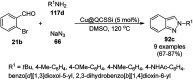


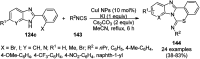

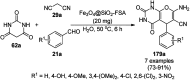
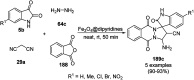

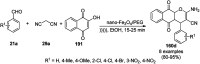

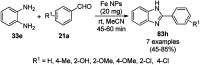

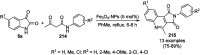
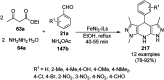
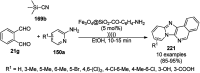
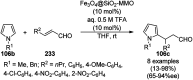
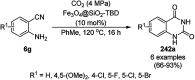
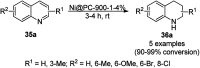

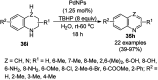

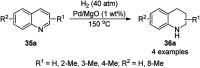


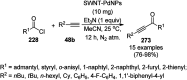



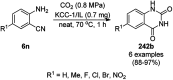

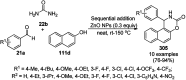

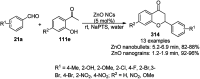
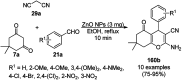


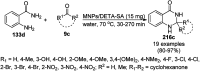
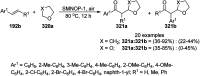

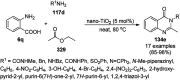
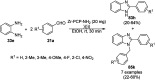
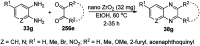
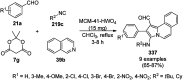




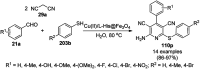

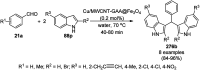


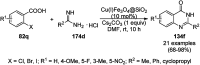
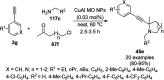



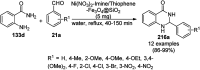

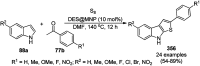


Similar articles
-
Alkynoates as Versatile and Powerful Chemical Tools for the Rapid Assembly of Diverse Heterocycles under Transition-Metal Catalysis: Recent Developments and Challenges.Top Curr Chem (Cham). 2021 Jan 5;379(1):3. doi: 10.1007/s41061-020-00316-4. Top Curr Chem (Cham). 2021. PMID: 33398642 Review.
-
Ring-strain-enabled reaction discovery: new heterocycles from bicyclo[1.1.0]butanes.Acc Chem Res. 2015 Apr 21;48(4):1149-58. doi: 10.1021/ar500437h. Epub 2015 Mar 16. Acc Chem Res. 2015. PMID: 25775119
-
Copper-Catalyzed Oxidative Carbon-Carbon and/or Carbon-Heteroatom Bond Formation with O2 or Internal Oxidants.Acc Chem Res. 2018 May 15;51(5):1092-1105. doi: 10.1021/acs.accounts.7b00611. Epub 2018 Apr 12. Acc Chem Res. 2018. PMID: 29648789
-
Catalysis by metal nanoparticles embedded on metal-organic frameworks.Chem Soc Rev. 2012 Aug 7;41(15):5262-84. doi: 10.1039/c2cs35047e. Epub 2012 Jun 13. Chem Soc Rev. 2012. PMID: 22695806
-
Transition metal-free one-pot synthesis of nitrogen-containing heterocycles.Mol Divers. 2016 Feb;20(1):185-232. doi: 10.1007/s11030-015-9596-0. Epub 2015 Jun 9. Mol Divers. 2016. PMID: 26055184 Review.
Cited by
-
Synthesis and characterization of new 1,4-dihydropyran derivatives by novel Ta-MOF nanostructures as reusable nanocatalyst with antimicrobial activity.Front Chem. 2022 Sep 27;10:967111. doi: 10.3389/fchem.2022.967111. eCollection 2022. Front Chem. 2022. PMID: 36238096 Free PMC article.
-
1,3-Dipolar cycloaddition reactions of isatin-derived azomethine ylides for the synthesis of spirooxindole and indole-derived scaffolds: recent developments.Mol Divers. 2023 Oct;27(5):2365-2397. doi: 10.1007/s11030-022-10510-9. Epub 2022 Aug 4. Mol Divers. 2023. PMID: 35925529 Review.
-
A novel magnetic FSM-16 supported ionic liquid/Pd complex as a high performance and recyclable catalyst for the synthesis of pyrano[3,2-c]chromenes.RSC Adv. 2024 May 22;14(23):16401-16410. doi: 10.1039/d4ra01381f. eCollection 2024 May 15. RSC Adv. 2024. PMID: 38779385 Free PMC article.
-
Photocatalytic dye degradation and photoexcited anti-microbial activities of green zinc oxide nanoparticles synthesized via Sargassum muticum extracts.RSC Adv. 2022 Jan 5;12(2):985-997. doi: 10.1039/d1ra08196a. eCollection 2021 Dec 22. RSC Adv. 2022. PMID: 35425145 Free PMC article.
-
MgFe2O4@Tris magnetic nanoparticles: an effective and powerful catalyst for one-pot synthesis of pyrazolopyranopyrimidine and tetrahydrodipyrazolopyridine derivatives.RSC Adv. 2024 Feb 15;14(9):6006-6015. doi: 10.1039/d3ra07934a. eCollection 2024 Feb 14. RSC Adv. 2024. PMID: 38362071 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

