A novel highly selective electrochemical chlorobenzene sensor based on ternary oxide RuO2/ZnO/TiO2 nanocomposites
- PMID: 35516515
- PMCID: PMC9056640
- DOI: 10.1039/d0ra05824f
A novel highly selective electrochemical chlorobenzene sensor based on ternary oxide RuO2/ZnO/TiO2 nanocomposites
Abstract
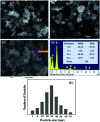
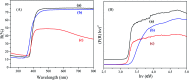
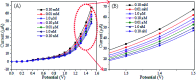
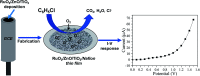
A novel electrochemical (EC) chlorobenzene (CBZ) sensor was fabricated using a ternary oxide RuO2/ZnO/TiO2 nanocomposite (NC)-decorated glassy carbon electrode (GCE). The nanoparticles (NPs) were synthesized by a wet-chemical method and characterized by X-ray photoelectron spectroscopy (XPS), powder X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), energy-dispersive X-ray spectroscopy (EDS), and ultraviolet-visible (UV-vis) spectroscopy. The synthesized RuO2/ZnO/TiO2 NC was layered as thin film on a GCE with Nafion (5% suspension in ethanol) adhesive, and the as-prepared sensor was subjected to CBZ analysis using an electrochemical approach. The calibration of the proposed CBZ sensor was executed with a linear relation of current versus concentration of CBZs known as the calibration curve. The sensitivity (32.02 μA μM-1 cm-2) of the CBZ sensor was calculated from the slope of the calibration curve by considering the active surface area of the GCE (0.0316 cm2). The lower detection limit (LD; 98.70 ± 4.90 pM) was also calculated at a signal-to-noise ratio of 3. Besides these, the response current followed a linear relationship with the concentration of chlorobenzene and the linear dynamic range (LDR) was denoted in the range of 0.1 nM to 1.0 μM. Moreover, the CBZ sensor was found to exhibit good reproducibility, reliability, stability, and fast response time. Finally, the sensing mechanism was also discussed with the energy-band theory of ternary doped semiconductor materials. The sensing activity of the proposed sensor was significantly enhanced due to the combined result of depletion layer formation at the heterojunction of RuO2/ZnO/TiO2 NCs as well as the activity of RuO2 NPs as oxidation catalysts. The proposed CBZ sensor probe based on ternary oxide RuO2/ZnO/TiO2 NCs was developed with significant analytical parameters for practical application in monitoring the environmental pollutants of CBZs for the safety of environmental fields on a large scale.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
On behalf of all authors, the corresponding author declares that there is no conflict of interest.
Figures







Similar articles
-
An Electrochemical Approach for the Selective Detection of Cancer Metabolic Creatine Biomarker with Porous Nano-Formulated CMNO Materials Decorated Glassy Carbon Electrode.Sensors (Basel). 2020 Dec 10;20(24):7060. doi: 10.3390/s20247060. Sensors (Basel). 2020. PMID: 33321693 Free PMC article.
-
Sensitive Electrochemical Detection of 4-Nitrophenol with PEDOT:PSS Modified Pt NPs-Embedded PPy-CB@ZnO Nanocomposites.Biosensors (Basel). 2022 Nov 8;12(11):990. doi: 10.3390/bios12110990. Biosensors (Basel). 2022. PMID: 36354499 Free PMC article.
-
Wet-chemically prepared low-dimensional ZnO/Al2O3/Cr2O3 nanoparticles for xanthine sensor development using an electrochemical method.RSC Adv. 2018 Apr 3;8(23):12562-12572. doi: 10.1039/c8ra01734d. eCollection 2018 Apr 3. RSC Adv. 2018. PMID: 35541273 Free PMC article.
-
An alternative electrochemical approach for toluene detection with ZnO/MgO/Cr2O3 nanofibers on a glassy carbon electrode for environmental monitoring.RSC Adv. 2020 Dec 17;10(73):44641-44653. doi: 10.1039/d0ra08577d. eCollection 2020 Dec 17. RSC Adv. 2020. PMID: 35516258 Free PMC article.
-
Electrochemical sensing of nicotine using CuWO4 decorated reduced graphene oxide immobilized glassy carbon electrode.Ultrason Sonochem. 2019 Jul;55:196-206. doi: 10.1016/j.ultsonch.2019.01.038. Epub 2019 Jan 29. Ultrason Sonochem. 2019. PMID: 30878204 Review.
Cited by
-
Integrated pH Sensors Based on RuO2/GO Nanocomposites Fabricated Using the Aerosol Jet Printing Method.ACS Omega. 2023 Nov 29;8(49):46794-46803. doi: 10.1021/acsomega.3c06309. eCollection 2023 Dec 12. ACS Omega. 2023. PMID: 38107955 Free PMC article.
-
Sensitive Detection of Thiourea Hazardous Toxin with Sandwich-Type Nafion/CuO/ZnO Nanospikes/Glassy Carbon Composite Electrodes.Polymers (Basel). 2021 Nov 19;13(22):3998. doi: 10.3390/polym13223998. Polymers (Basel). 2021. PMID: 34833297 Free PMC article.
-
Dielectric properties of poly-(3-octylthiophene) thin films mixed with oleic acid capped cadmium selenide nanoparticles.RSC Adv. 2020 Dec 22;10(73):45139-45148. doi: 10.1039/d0ra09236c. eCollection 2020 Dec 17. RSC Adv. 2020. PMID: 35516243 Free PMC article.
References
-
- Kometani N. Inata S. Shimokawa A. Yonezawa Y. Int. J. Photoenergy. 2008:512170. doi: 10.1155/2008/512170. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

