All-solid-state flexible supercapacitor based on nanotube-reinforced polypyrrole hollowed structures
- PMID: 35516535
- PMCID: PMC9057791
- DOI: 10.1039/d0ra08064k
All-solid-state flexible supercapacitor based on nanotube-reinforced polypyrrole hollowed structures
Abstract
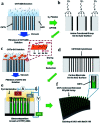
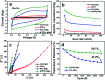
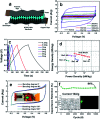
Supercapacitors are strong future candidates for energy storage devices owing to their high power density, fast charge-discharge rate, and long cycle stability. Here, a flexible supercapacitor with a large specific capacitance of 443 F g-1 at a scan rate of 2 mV s-1 is demonstrated using nanotube-reinforced polypyrrole nanowires with hollowed cavities grown vertically on a nanotube/graphene based film. Using these electrodes, we obtain improved capacitance, rate capability, and cycle stability for over 3000 cycles. The assembled all-solid-state supercapacitor exhibits excellent mechanical flexibility, with the capacity to endure a 180° bending angle along with a maximum specific and volumetric energy density of 7 W h kg-1 (8.2 mW h cm-3) at a power density of 75 W kg-1 (0.087 W cm-3), and it showed an energy density of 4.13 W h kg-1 (4.82 mW h cm-3) even at a high power density of 3.8 kW kg-1 (4.4 W cm-3). Also, it demonstrates a high cycling stability of 94.3% after 10 000 charge/discharge cycles at a current density of 10 A g-1. Finally, a foldable all-solid-state supercapacitor is demonstrated, which confirms the applicability of the reported supercapacitor for use in energy storage devices for future portable, foldable, or wearable electronics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Flexible all-solid-state supercapacitors based on polyaniline orderly nanotubes array.Nanoscale. 2017 Jan 7;9(1):193-200. doi: 10.1039/c6nr07921k. Epub 2016 Dec 1. Nanoscale. 2017. PMID: 27906390
-
In Situ Growth of a High-Performance All-Solid-State Electrode for Flexible Supercapacitors Based on a PANI/CNT/EVA Composite.Polymers (Basel). 2019 Jan 21;11(1):178. doi: 10.3390/polym11010178. Polymers (Basel). 2019. PMID: 30960162 Free PMC article.
-
Fabrication of a High-Energy Flexible All-Solid-State Supercapacitor Using Pseudocapacitive 2D-Ti3C2Tx-MXene and Battery-Type Reduced Graphene Oxide/Nickel-Cobalt Bimetal Oxide Electrode Materials.ACS Appl Mater Interfaces. 2020 Nov 25;12(47):52749-52762. doi: 10.1021/acsami.0c16221. Epub 2020 Nov 13. ACS Appl Mater Interfaces. 2020. PMID: 33185100
-
All-solid-state flexible supercapacitors based on highly dispersed polypyrrole nanowire and reduced graphene oxide composites.ACS Appl Mater Interfaces. 2014 Oct 22;6(20):17937-43. doi: 10.1021/am5059603. Epub 2014 Oct 6. ACS Appl Mater Interfaces. 2014. PMID: 25247315
-
High-performance MnO2-deposited graphene/activated carbon film electrodes for flexible solid-state supercapacitor.Sci Rep. 2017 Oct 9;7(1):12857. doi: 10.1038/s41598-017-11267-0. Sci Rep. 2017. PMID: 28993627 Free PMC article.
Cited by
-
Gels in Motion: Recent Advancements in Energy Applications.Gels. 2024 Feb 2;10(2):122. doi: 10.3390/gels10020122. Gels. 2024. PMID: 38391452 Free PMC article. Review.
References
-
- Zhang K. Zhang L. L. Zhao X. S. Wu J. Chem. Mater. 2010;22:1392–1401. doi: 10.1021/cm902876u. - DOI
LinkOut - more resources
Full Text Sources

