A functional modified graphene oxide/nanodiamond/nano zinc oxide composite for excellent vulcanization properties of natural rubber
- PMID: 35516552
- PMCID: PMC9057914
- DOI: 10.1039/d0ra07404g
A functional modified graphene oxide/nanodiamond/nano zinc oxide composite for excellent vulcanization properties of natural rubber
Abstract
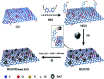
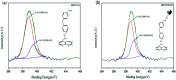
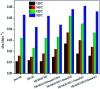
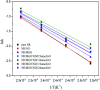
A modified graphene oxide/nanodiamond/nanozinc oxide (MGO/ND/nanoZnO) functional hybrid filler is designed and prepared to improve the vulcanization efficiency of a rubber composite and to reduce the use of ZnO. ND was grafted onto graphite oxide with the aid of 4,4'-methylene diphenyl diisocyanate (MDI). NanoZnO, with high surface activity, was then loaded onto the MGO/ND complex through the wet chemical method, in order to synthesize the MGO/ND/nanoZnO functional hybrid filler. Rubber composites were prepared using the rubber latex composite method and their vulcanization behaviors were investigated. Our results show that the MGO/ND/nanoZnO functional hybrid filler can remarkably improve the vulcanization behaviors of the rubber composite. Compared with that of pure natural rubber (NR), the vulcanization activation energy of the rubber composite was reduced by approximately 16%. Moreover, the vulcanization efficiency can be improved by 63% (i.e., the optimum cure time is shortened from the original 405 s to 150 s) after the same amount of traditional ZnO was replaced by the functional hybrid filler loaded with 1 wt% nanoZnO. The prepared MGO/ND/nanoZnO functional hybrid filler thus provides a promising alternative to improve the vulcanization efficiency of rubber composites.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures








Similar articles
-
Enhancing Rubber Vulcanization Cure Kinetics: Lowering Vulcanization Temperature by Addition of MgO as Co-Cure Activator in ZnO-Based Cure Activator Systems.Polymers (Basel). 2024 Mar 22;16(7):876. doi: 10.3390/polym16070876. Polymers (Basel). 2024. PMID: 38611134 Free PMC article.
-
Advances in Rubber Compounds Using ZnO and MgO as Co-Cure Activators.Polymers (Basel). 2022 Dec 3;14(23):5289. doi: 10.3390/polym14235289. Polymers (Basel). 2022. PMID: 36501682 Free PMC article.
-
Enhancing the Performance of Rubber with Nano ZnO as Activators.ACS Appl Mater Interfaces. 2020 Oct 21;12(42):48007-48015. doi: 10.1021/acsami.0c15114. Epub 2020 Oct 11. ACS Appl Mater Interfaces. 2020. PMID: 33040537
-
Interfacial Adhesion in Silica-Silane Filled NR Composites: A Short Review.Polymers (Basel). 2022 Jul 1;14(13):2705. doi: 10.3390/polym14132705. Polymers (Basel). 2022. PMID: 35808750 Free PMC article. Review.
-
Synthetic Polyisoprene Rubber as a Mimic of Natural Rubber: Recent Advances on Synthesis, Nanocomposites, and Applications.Polymers (Basel). 2023 Oct 13;15(20):4074. doi: 10.3390/polym15204074. Polymers (Basel). 2023. PMID: 37896318 Free PMC article. Review.
Cited by
-
Preparation of Zinc Oxide with Core-Shell Structure and Its Application in Rubber Products.Polymers (Basel). 2023 May 18;15(10):2353. doi: 10.3390/polym15102353. Polymers (Basel). 2023. PMID: 37242928 Free PMC article.
-
Enhancing Rubber Vulcanization Cure Kinetics: Lowering Vulcanization Temperature by Addition of MgO as Co-Cure Activator in ZnO-Based Cure Activator Systems.Polymers (Basel). 2024 Mar 22;16(7):876. doi: 10.3390/polym16070876. Polymers (Basel). 2024. PMID: 38611134 Free PMC article.
-
Effects of mixing temperature on the extrusion rheological behaviors of rubber-based compounds.RSC Adv. 2021 Nov 4;11(56):35703-35710. doi: 10.1039/d1ra05929g. eCollection 2021 Oct 28. RSC Adv. 2021. PMID: 35493166 Free PMC article.
-
Graphene-Based Hybrid Fillers for Rubber Composites.Molecules. 2024 Feb 26;29(5):1009. doi: 10.3390/molecules29051009. Molecules. 2024. PMID: 38474521 Free PMC article. Review.
-
On the In Vitro and In Vivo Hazard Assessment of a Novel Nanomaterial to Reduce the Use of Zinc Oxide in the Rubber Vulcanization Process.Toxics. 2022 Dec 13;10(12):781. doi: 10.3390/toxics10120781. Toxics. 2022. PMID: 36548614 Free PMC article.
References
-
- Mark J. E., Erman B. and Roland M., The science and technology of rubber, Academic press, 2013
-
- Lopez L. M. Cosgrove A. B. Hernandez-Ortiz J. P. Osswald T. A. Polym. Eng. Sci. 2007;47:675–683.
-
- Coran A. Y. Rubber Chem. Technol. 1965;38:1–14.
-
- Ehabé E. Bonfils F. Aymard C. Akinlabi A. K. Sainte Beuve J. Polym. Test. 2005;24:620–627. doi: 10.1016/j.polymertesting.2005.03.006. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

