Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids
- PMID: 35516563
- PMCID: PMC9057921
- DOI: 10.1039/d0ra06642g
Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids
Abstract
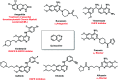
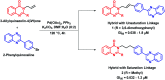
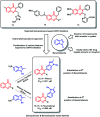
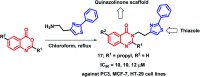
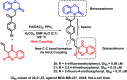
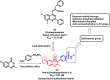
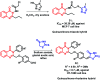
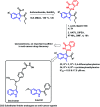
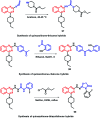
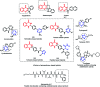
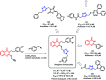
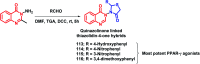
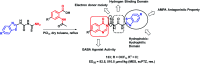
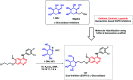
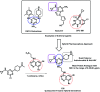
Due to the pharmacological activities of quinazoline and quinazolinone scaffolds, it has aroused great interest in medicinal chemists for the development of new drugs or drug candidates. The pharmacological activities of quinazoline and its related scaffolds include anti-cancer, anti-microbial, anti-convulsant, and antihyperlipidaemia. Recently, molecular hybridization technology is used for the development of hybrid analogues with improved potency by combining two or more pharmacophores of bioactive scaffolds. The molecular hybridization of various biologically active pharmacophores with quinazoline derivatives resulted in lead compounds with multi-faceted biological activity wherein specific as well as multiple targets were involved. The present review summarizes the advances in lead compounds of quinazoline hybrids and their related heterocycles in medicinal chemistry. Moreover, the review also helps to intensify the drug development process by providing an understanding of the potential role of these hybridized pharmacophoric features in exhibiting various pharmacological activities.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict of interest among the authors.
Figures































Similar articles
-
A literature review on pharmacological aspects, docking studies, and synthetic approaches of quinazoline and quinazolinone derivatives.Arch Pharm (Weinheim). 2024 Aug;357(8):e2400057. doi: 10.1002/ardp.202400057. Epub 2024 May 22. Arch Pharm (Weinheim). 2024. PMID: 38775630 Review.
-
Quinazoline and quinazolinone as important medicinal scaffolds: a comparative patent review (2011-2016).Expert Opin Ther Pat. 2018 Apr;28(4):281-297. doi: 10.1080/13543776.2018.1432596. Epub 2018 Feb 5. Expert Opin Ther Pat. 2018. PMID: 29368977 Review.
-
Quinazolinone-based hybrids with diverse biological activities: A mini-review.J Res Med Sci. 2022 Sep 27;27:68. doi: 10.4103/jrms.jrms_1025_21. eCollection 2022. J Res Med Sci. 2022. PMID: 36353342 Free PMC article. Review.
-
Development of Phenothiazine Hybrids with Potential Medicinal Interest: A Review.Molecules. 2022 Jan 3;27(1):276. doi: 10.3390/molecules27010276. Molecules. 2022. PMID: 35011508 Free PMC article. Review.
-
An Insight on the Prospect of Quinazoline and Quinazolinone Derivatives as Anti-tubercular Agents.Curr Org Synth. 2023;20(8):838-869. doi: 10.2174/1570179420666230316094435. Curr Org Synth. 2023. PMID: 36927421 Review.
Cited by
-
Synthesis and preliminary structure-activity relationship study of 3-methylquinazolinone derivatives as EGFR inhibitors with enhanced antiproliferative activities against tumour cells.J Enzyme Inhib Med Chem. 2021 Dec;36(1):1205-1216. doi: 10.1080/14756366.2021.1933466. J Enzyme Inhib Med Chem. 2021. PMID: 34074193 Free PMC article.
-
Application of Deep Eutectic Solvents in the Synthesis of Substituted 2-Mercaptoquinazolin-4(3H)-Ones: A Comparison of Selected Green Chemistry Methods.Molecules. 2022 Jan 16;27(2):558. doi: 10.3390/molecules27020558. Molecules. 2022. PMID: 35056873 Free PMC article.
-
Computer-Aided Design and Synthesis of (Functionalized quinazoline)-(α-substituted coumarin)-arylsulfonate Conjugates against Chikungunya Virus.Int J Mol Sci. 2022 Jul 11;23(14):7646. doi: 10.3390/ijms23147646. Int J Mol Sci. 2022. PMID: 35886992 Free PMC article.
-
Diversity-oriented synthesis of 1H-1,2,3-triazole tethered pyrazolo[5,1-b]quinazoline hybrids as antimicrobial agents.Mol Divers. 2024 Oct;28(5):2875-2896. doi: 10.1007/s11030-023-10721-8. Epub 2023 Sep 11. Mol Divers. 2024. PMID: 37697023
-
Synthesis and Biological Evaluation of 2-Substituted Quinazolin-4(3H)-Ones with Antiproliferative Activities.Molecules. 2023 Dec 2;28(23):7912. doi: 10.3390/molecules28237912. Molecules. 2023. PMID: 38067641 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

