Widely used catalysts in biodiesel production: a review
- PMID: 35516564
- PMCID: PMC9058015
- DOI: 10.1039/d0ra07931f
Widely used catalysts in biodiesel production: a review
Abstract
An ever-increasing energy demand and environmental problems associated with exhaustible fossil fuels have led to the search for an alternative renewable source of energy. In this context, biodiesel has attracted attention worldwide as an eco-friendly alternative to fossil fuel for being renewable, non-toxic, biodegradable, and carbon-neutral. Although the homogeneous catalyst has its own merits, much attention is currently paid toward the chemical synthesis of heterogeneous catalysts for biodiesel production as it can be tuned as per specific requirement and easily recovered, thus enhancing reusability. Recently, biomass-derived heterogeneous catalysts have risen to the forefront of biodiesel productions because of their sustainable, economical and eco-friendly nature. Furthermore, nano and bifunctional catalysts have emerged as a powerful catalyst largely due to their high surface area, and potential to convert free fatty acids and triglycerides to biodiesel, respectively. This review highlights the latest synthesis routes of various types of catalysts (including acidic, basic, bifunctional and nanocatalysts) derived from different chemicals, as well as biomass. In addition, the impacts of different methods of preparation of catalysts on the yield of biodiesel are also discussed in details.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









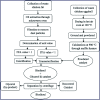


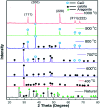
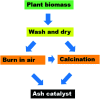


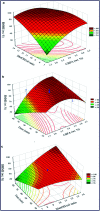











References
-
- Yin X. Duan X. You Q. Dai C. Tan Z. Zhu X. Energy Convers. Manage. 2016;112:199–207. doi: 10.1016/j.enconman.2016.01.026. - DOI
-
- International Renewable Energy Agency (IRENA), Global Energy Transformation: A Roadmap to 2050, 2018
-
- IEA, Int. Energy Agency, Peris, 2016, pp. 1–77
-
- Kulkarni M. G. Dalai A. K. Ind. Eng. Chem. Res. 2006;45:2901–2913. doi: 10.1021/ie0510526. - DOI
Publication types
LinkOut - more resources
Full Text Sources

