Post immunization COVID-19 disease
- PMID: 35516695
- PMCID: PMC9067203
- DOI: 10.4103/jfmpc.jfmpc_1975_21
Post immunization COVID-19 disease
Abstract
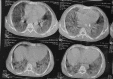
One hundred and two patients reported to peripheral hospital, of these 73 (77%) and 23 (95%) suffered from COVID-19 disease, were immunized as per availability of Covishield 78 (77%) and Covaxin 24 (23%), respectively. Of these, three died (3%). Inflammatory markers were raised in Covishield vs. Covaxin 26 (36%) vs. 7 (31%), 46 (63%) vs. 8 (35%), 57 (78%) vs. 14 (61%), 29 (40%) vs. (73%), C-reactive proteins and serum ferritin, and positive for COVID-19 antigen and RTPCR and COVID-19 pneumonia, respectively. Irrespective of immunization status of victim with symptoms should be investigated for possibility of severe acute respiratory syndrome COVID-beta 2 (SARS-CoV-2) virus infection. These findings confirmed the need for a Booster dose of COVID-19 vaccination.
Keywords: COVID-19; HRCT; vaccine.
Copyright: © 2022 Journal of Family Medicine and Primary Care.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–6. - PubMed
-
- Wise J. Covid-19:European countries suspend use of Oxford –AstraZeneca vaccine after report of blood clot. BMJ. 2021;372:n699. - PubMed
-
- Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody –dependent enhancement and SARS-cov-2 vaccines and therapies. Nat Microbiol. 2020;5:1185–91. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous