An electrochemical sensor based on copper-based metal-organic framework-reduced graphene oxide composites for determination of 2,4-dichlorophenol in water
- PMID: 35516768
- PMCID: PMC9057864
- DOI: 10.1039/d0ra06700h
An electrochemical sensor based on copper-based metal-organic framework-reduced graphene oxide composites for determination of 2,4-dichlorophenol in water
Abstract
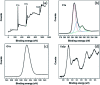
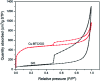
In the present work, we reported the fabrication of a novel electrochemical sensing platform to detect 2,4-dichlorophenol (2,4-DCP) by using a copper benzene-1,3,5-tricarboxylate-graphene oxide (Cu-BTC/GO) composite. The sensor was prepared by drop-casting Cu-BTC/GO suspension onto the electrode surface followed by electrochemical reduction, leading to the generation of an electrochemically reduced graphene oxide network (ErGO). By combining the large specific area of the Cu-BTC matrix with the electrical percolation from the graphene network, the number of accessible reaction sites was strongly increased, which consequently improved the detection performance. The electrochemical characteristics of the composite were revealed by cyclic voltammetry and electrochemical impedance spectroscopy. For the detection of 2,4-DCP, differential pulse voltammetry was used to emphasize the faradaic reaction related to the oxidation of the analyte. The results displayed a low detection limit (83 × 10-9 M) and a linear range from 1.5 × 10-6 M to 24 × 10-6 M alongside high reproducibility (RSD = 2.5% for eight independent sensors) and good stability. Importantly, the prepared sensors were sufficiently selective against interference from other pollutants in the same electrochemical window. Notably, the presented sensors have already proven their ability in detecting 2,4-DCP in real field samples with high accuracy (recovery range = 97.17-104.15%).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

