Folic acid-conjugated gold nanorod@polypyrrole@Fe3O4 nanocomposites for targeted MR/CT/PA multimodal imaging and chemo-photothermal therapy
- PMID: 35516886
- PMCID: PMC9065171
- DOI: 10.1039/c9ra00541b
Folic acid-conjugated gold nanorod@polypyrrole@Fe3O4 nanocomposites for targeted MR/CT/PA multimodal imaging and chemo-photothermal therapy
Abstract
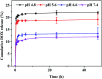
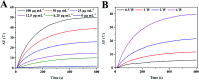
Integrating multimodal bioimaging and different therapies into one nanoplatform is a promising strategy for biomedical applications, but remains a great challenge. Herein, we have synthesized a biocompatible folic acid (FA) functionalized gold nanorod@polypyrrole@Fe3O4 (GNR@PPy@Fe3O4-FA) nanocomposite through a facile method. The conjugated FA has endowed the nanocomposite with the ability to recognize targeted cancer cells. Importantly, the nanocomposite has been successfully utilized for magnetic resonance (MR), computed tomography (CT) and photoacoustic (PA) multimodal imaging. Moreover, the GNR@PPy@Fe3O4-DOX nanocomposite shows pH-responsive chemotherapy and enables the integration of photothermal therapy and chemotherapy to achieve superior antitumor efficacy. The GNR@PPy@Fe3O4-DOX nanocomposites have a drug release of 23.64%, and the photothermal efficiency of the GNR@PPy@Fe3O4 nanocomposites reaches 51.46%. Cell viability decreases to 15.83% and 16.47% because of the combination of chemo-photothermal therapy effects. Moreover, the GNR@PPy@Fe3O4-DOX-FA nanocomposite could target cancer cells via folic acid and under a magnetic field. The in vivo multimodal imaging and chemo-photothermal therapy effects showed that the GNR@PPy@Fe3O4-DOX-FA nanocomposites are a good contrast and theranostic agent. Thus, this multifunctional nanocomposite could be a promising theranostic platform for cancer diagnosis and therapy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
LinkOut - more resources
Full Text Sources

