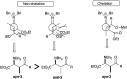
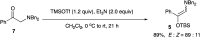Preparation and facile addition reactions of iminium salts derived from amino ketene silyl acetal and amino silyl enol ether
- PMID: 35516926
- PMCID: PMC9055599
- DOI: 10.1039/d0ra05768a
Preparation and facile addition reactions of iminium salts derived from amino ketene silyl acetal and amino silyl enol ether
Abstract
While iminium salts generated by the oxidation of amino ketene silyl acetals show intriguing reactivities to give useful γ-oxo-α-amino esters via reactions with silyl enol ethers in good yields, new iminium salts are also prepared by the oxidation of amino silyl enol ethers. They undergo facile addition reaction with various nucleophiles to give α-amino ketone derivatives in good yields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Synthesis of α,α-disubstituted α-amino esters: nucleophilic addition to iminium salts generated from amino ketene silyl acetals.J Org Chem. 2011 Dec 2;76(23):9670-7. doi: 10.1021/jo201692x. Epub 2011 Oct 31. J Org Chem. 2011. PMID: 22011263
-
Coupling Reaction of Enol Derivatives with Silyl Ketene Acetals Catalyzed by Gallium Trihalides.Chemistry. 2016 Aug 8;22(33):11837-45. doi: 10.1002/chem.201602150. Epub 2016 Jul 11. Chemistry. 2016. PMID: 27400389
-
Two Reaction Mechanisms via Iminium Ion Intermediates: The Different Reactivities of Diphenylprolinol Silyl Ether and Trifluoromethyl-Substituted Diarylprolinol Silyl Ether.Chemistry. 2015 Aug 24;21(35):12337-46. doi: 10.1002/chem.201500326. Epub 2015 Jun 19. Chemistry. 2015. PMID: 26096559
-
Rhenium-Catalyzed Decarboxylative Coupling of Cyclic Enol Carbonates with Silyl Enol Ethers and Ketene Silyl Acetals.Org Lett. 2023 Apr 7;25(13):2275-2279. doi: 10.1021/acs.orglett.3c00543. Epub 2023 Mar 23. Org Lett. 2023. PMID: 36951801
-
A new synthetic method for alpha-alkoxycarbonyl iminium salt and its reaction with nucleophiles.J Am Chem Soc. 2005 Mar 16;127(10):3296-7. doi: 10.1021/ja042330f. J Am Chem Soc. 2005. PMID: 15755144
References
-
-
For iminium ion cyclization, see:
- Corey E. J. Balanson R. D. J. Am. Chem. Soc. 1974;96:6516–6517. doi: 10.1021/ja00827a046. - DOI
- Yamada S. Konda M. Shioiri T. Tetrahedron Lett. 1972;13:2215–2218. doi: 10.1016/S0040-4039(01)84809-7. - DOI
-
. For N-acyl iminium ion cyclization, see
- Dijkink J. Shoemeker H. E. Speckamp W. N. Tetrahedron Lett. 1975;16:4043–4046. doi: 10.1016/S0040-4039(00)91231-0. - DOI
- Dijkink J. Speckamp W. N. Tetrahedron Lett. 1975;16:4047–4050. doi: 10.1016/S0040-4039(00)91232-2. - DOI
-
-
- Mooiweer H. H. Hiemstra H. Speckamp W. N. Tetrahedron. 1989;45:4627–4636. doi: 10.1016/S0040-4020(01)89098-0. - DOI
- Mooiweer H. H. Roots E. C. Hiemstra H. Speckamp W. N. J. Org. Chem. 1992;57:6769–6778. doi: 10.1021/jo00051a019. - DOI
- Okuda M. Hioki H. Miyagi W. Ito S. Tetrahedron Lett. 1993;34:6131–6134. doi: 10.1016/S0040-4039(00)61748-3. - DOI
- Yoshida J. Suga S. Okajima M. Tetrahedron Lett. 2001;42:2173–2176. doi: 10.1016/S0040-4039(01)00128-9. - DOI
- Yoshida J. Suga S. Watanabe M. J. Am. Chem. Soc. 2002;124:14824–14825. doi: 10.1021/ja028663z. - DOI - PubMed
- Knochel P. Millot N. Piazza C. Avolio S. Synthesis. 2002:941–944.
- Bieber L. W. Estevam I. H. S. Tetrahedron Lett. 2003;44:667–670. doi: 10.1016/S0040-4039(02)02667-9. - DOI
- Suginome M. Uehlin L. Murakami M. Org. Lett. 2004;6:1167–1169. doi: 10.1021/ol0497436. - DOI - PubMed
- Murai T. Mutoh Y. Ohta Y. Murakami M. J. Am. Chem. Soc. 2004;126:5968–5969. doi: 10.1021/ja048627v. - DOI - PubMed
- Suginome M. Uehlin L. Murakami M. J. Am. Chem. Soc. 2004;126:13196–13197. doi: 10.1021/ja045827y. - DOI - PubMed
-
-
For Petasis reactions including the synthesis of α-monosubstituted amino acids, see:
- Petasis N. A. Akritopoulou I. Tetrahedron Lett. 1993;34:583–586. doi: 10.1016/S0040-4039(00)61625-8. - DOI
- Petasis N. A. Zavialov I. A. J. Am. Chem. Soc. 1997;119:445–446. doi: 10.1021/ja963178n. - DOI
- Petasis N. A. Goodman A. Zavialov I. A. Tetrahedron. 1997;53:16463–16470. doi: 10.1016/S0040-4020(97)01028-4. - DOI
- Petasis N. A. Zavialov I. A. J. Am. Chem. Soc. 1998;120:11798–11799. doi: 10.1021/ja981075u. - DOI
- Petasis N. A. Boral S. Tetrahedron Lett. 2001;42:539–542. doi: 10.1016/S0040-4039(00)02014-1. - DOI
- Koolmeister T. Södelgren M. Scobie M. Tetrahedron Lett. 2002;43:5969–5970. doi: 10.1016/S0040-4039(02)01263-7. - DOI
- Jourdan H. Gouhier G. Hijfte L. V. Angibaud P. Piettre S. R. Tetrahedron Lett. 2005;46:8027–8031. doi: 10.1016/j.tetlet.2005.09.060. - DOI
- Southwood T. J. C Curry M. Hutton C. A. Tetrahedron. 2006;62:236–242. doi: 10.1016/j.tet.2005.09.114. - DOI
-
. For asymmetric Petasis reaction:
- Lou S. Schaus S. E. J. Am. Chem. Soc. 2008;130:6922–6923. doi: 10.1021/ja8018934. - DOI - PMC - PubMed
-
-
- Niwa Y. Shimizu M. J. Am. Chem. Soc. 2003;125:3720–3721. doi: 10.1021/ja029639o. - DOI - PubMed
- Shimizu M. Itou H. Miura M. J. Am. Chem. Soc. 2005;127:3296–3297. doi: 10.1021/ja042330f. - DOI - PubMed
- Iwao T. Shimizu M. Heterocycles. 2009;77:767–772. doi: 10.3987/COM-08-S(F)89. - DOI
- Shimizu M. Itou H. Iwao T. Umeda Y. Chem. Lett. 2009;38:732–733. doi: 10.1246/cl.2009.732. - DOI
- Shimizu M. Hachiya I. Mizota I. Chem. Commun. 2009:874–889. doi: 10.1039/B814930E. - DOI - PubMed
- Hata S. Koyama H. Shimizu M. J. Org. Chem. 2011;76:9670–9677. doi: 10.1021/jo201692x. - DOI - PubMed
- Shimizu M. Kusunoki T. Yoshida M. Kondo K. Mizota I. Chem. Lett. 2011;40:351–353. doi: 10.1246/cl.2011.351. - DOI
- Mizota I. Shimizu M. Chem. Rec. 2016;16:688–702. doi: 10.1002/tcr.201500267. - DOI - PubMed
- Shimizu M. Imazato H. Mizota I. Zhu Y. RSC Adv. 2019;9:17341–17346. doi: 10.1039/C9RA02204J. - DOI - PMC - PubMed
- Shimizu M. Mushika M. Mizota I. Zhu Y. RSC Adv. 2019;9:23400–23407. doi: 10.1039/C9RA04889H. - DOI - PMC - PubMed
- Shimizu M. Furukawa Y. Mizota I. Zhu Y. New J. Chem. 2020;44:152–161. doi: 10.1039/C9NJ05114G. - DOI
- Shimizu M. Morimoto T. Yanagi Y. Mizota I. Zhu Y. RSC Adv. 2020;10:9955–9963. doi: 10.1039/D0RA01152E. - DOI - PMC - PubMed
-
-
We have already reported the formation of the iminium salt by 1H and 13C NMR spectra and a possible ionic mechanism by the ineffectiveness of radical scavengers.
-
LinkOut - more resources
Full Text Sources