Density functional study on the CO oxidation reaction mechanism on MnN2-doped graphene
- PMID: 35516928
- PMCID: PMC9055664
- DOI: 10.1039/d0ra05287f
Density functional study on the CO oxidation reaction mechanism on MnN2-doped graphene
Abstract
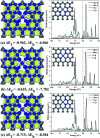
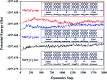
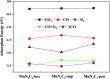
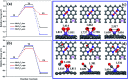
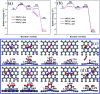
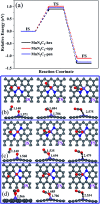
The CO oxidation mechanisms over three different MnN2-doped graphene (MnN2C2: MnN2C2-hex, MnN2C2-opp, MnN2C2-pen) structures were investigated through first-principles calculations. The vacancy in graphene can strongly stabilize Mn atoms and make them positively charged, which promotes O2 activation and weakens CO adsorption. Hence, CO oxidation activity is enhanced and the catalyst is prevented from being poisoned. CO oxidation reaction (COOR) on MnN2C2 along the Eley-Rideal (ER) mechanism and the Langmuir-Hinshelwood (LH) mechanism will leave one O atom on the Mn atom, which is difficult to react with isolated CO. COOR on MnN2C2-opp along the ER mechanism and termolecular Eley-Rideal (TER) mechanism need overcome low energy barriers in the rate limiting step (RLS), which are 0.544 and 0.342 eV, respectively. The oxidation of CO along TER mechanism on MnN2C2-opp is the best reaction pathway with smallest energy barrier. Therefore, the MnN2C2-opp is an efficient catalysis and this study has a guiding role in designing effective catalyst for CO oxidation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Role of sulfur vacancies in MoS2 monolayers in stabilizing Co atoms for efficient CO oxidation.RSC Adv. 2022 Nov 4;12(49):31525-31534. doi: 10.1039/d2ra06261e. eCollection 2022 Nov 3. RSC Adv. 2022. PMID: 36380960 Free PMC article.
-
Single Pt atom supported on penta-graphene as an efficient catalyst for CO oxidation.Phys Chem Chem Phys. 2019 Jun 21;21(23):12201-12208. doi: 10.1039/c9cp02306b. Epub 2019 Jun 3. Phys Chem Chem Phys. 2019. PMID: 31155626
-
First Principles Study on the CO Oxidation on Mn-Embedded Divacancy Graphene.Front Chem. 2018 May 29;6:187. doi: 10.3389/fchem.2018.00187. eCollection 2018. Front Chem. 2018. PMID: 29911100 Free PMC article.
-
Catalytic CO oxidation on B-doped and BN co-doped penta-graphene: a computational study.Phys Chem Chem Phys. 2018 Nov 7;20(41):26414-26421. doi: 10.1039/c8cp04745f. Epub 2018 Oct 11. Phys Chem Chem Phys. 2018. PMID: 30306166
-
Practical Considerations for Understanding Surface Reaction Mechanisms Involved in Heterogeneous Catalysis.ACS Catal. 2024 Oct 30;14(22):16770-16784. doi: 10.1021/acscatal.4c05188. eCollection 2024 Nov 15. ACS Catal. 2024. PMID: 39569155 Free PMC article. Review.
Cited by
-
Metal oxide adsorption on fullerene C60 and its potential for adsorption of pollutant gases; density functional theory studies.RSC Adv. 2021 May 12;11(28):17377-17390. doi: 10.1039/d1ra02251b. eCollection 2021 May 6. RSC Adv. 2021. PMID: 35479706 Free PMC article.
References
-
- Alavi A. Hu P. Deutsch T. Silvestrelli P. L. Hutter J. Phys. Rev. Lett. 1998;80:3650–3653. doi: 10.1103/PhysRevLett.80.3650. - DOI
LinkOut - more resources
Full Text Sources

