Hydrogenation of furfural by noble metal-free nickel modified tungsten carbide catalysts
- PMID: 35516944
- PMCID: PMC9055482
- DOI: 10.1039/d0ra02003f
Hydrogenation of furfural by noble metal-free nickel modified tungsten carbide catalysts
Abstract
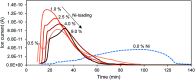
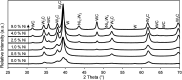
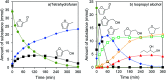
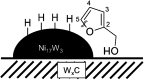
Nickel-tungsten carbide catalysts convert furfural to high value products in a liquid phase catalytic reaction. The product distribution depends on the solvent and the Ni-W-ratio of the catalyst. In isopropyl alcohol a combination of Ni and W x C enables the opening of the furan ring to yield 1,2-pentanediol. Nickel accelerates the tungsten oxide reduction in the tungsten carbide catalyst synthesis and facilitates the carbon insertion. Nickel modified tungsten carbide is a promising, noble metal-free catalyst system for the upgrading of furfural based renewable resources. Its preparation is facilitated compared to unmodified tungsten carbide catalysts.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Nickel-tungsten carbide catalysts for the production of 2,5-dimethylfuran from biomass-derived molecules.ChemSusChem. 2014 Apr;7(4):1068-72. doi: 10.1002/cssc.201301356. Epub 2014 Feb 26. ChemSusChem. 2014. PMID: 24574062
-
Design and Application of Mesoporous Catalysts for Liquid-Phase Furfural Hydrogenation.Molecules. 2025 Mar 12;30(6):1270. doi: 10.3390/molecules30061270. Molecules. 2025. PMID: 40142046 Free PMC article. Review.
-
Tungsten Carbide/Tungsten Oxide Catalysts for Efficient Electrocatalytic Hydrogen Evolution.Molecules. 2024 Dec 29;30(1):84. doi: 10.3390/molecules30010084. Molecules. 2024. PMID: 39795141 Free PMC article.
-
Influence of Highly Stable Ni2+ Species in Ni Phyllosilicate Catalysts on Selective Hydrogenation of Furfural to Furfuryl Alcohol.ACS Omega. 2022 Sep 8;8(1):249-261. doi: 10.1021/acsomega.2c03590. eCollection 2023 Jan 10. ACS Omega. 2022. PMID: 36643509 Free PMC article.
-
A Comprehensive Review on Metal Catalysts for the Production of Cyclopentanone Derivatives from Furfural and HMF.Molecules. 2023 Jul 14;28(14):5397. doi: 10.3390/molecules28145397. Molecules. 2023. PMID: 37513268 Free PMC article. Review.
References
-
- Biradar N. S. Hengne A. M. Birajdar S. N. Niphadkar P. S. Joshi P. N. Rode C. V. ACS Sustainable Chem. Eng. 2014;2:272–281. doi: 10.1021/sc400302b. - DOI
-
- Yan K. Wu G. Lafleur T. Jarvis C. Renewable Sustainable Energy Rev. 2014;38:663–676. doi: 10.1016/j.rser.2014.07.003. - DOI
-
- Mizugaki T. Yamakawa T. Nagatsu Y. Maeno Z. Mitsudome T. Jitsukawa K. Kaneda K. ACS Sustainable Chem. Eng. 2014;2:2243–2247. doi: 10.1021/sc500325g. - DOI
-
- Siegmeier R., Prescher G. and Maurer H., European Pat., 0,257,243, 1987
LinkOut - more resources
Full Text Sources

