Fast, efficient and clean adsorption of bisphenol-A using renewable mesoporous silica nanoparticles from sugarcane waste ash
- PMID: 35516950
- PMCID: PMC9055659
- DOI: 10.1039/d0ra05198e
Fast, efficient and clean adsorption of bisphenol-A using renewable mesoporous silica nanoparticles from sugarcane waste ash
Abstract
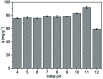
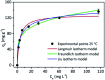
Even with all the biological problems associated with bisphenol-A (BPA), this chemical is still being widely used, especially in thermal paper receipts. In this study, renewable mesoporous silica nanoparticles (MSN), obtained from sugarcane ash, functionalized with hexadecyltrimethylammonium (CTAB) were applied as an adsorbent in the removal of BPA from the aqueous solution. The versatility of this material and its BPA adsorption capacity were tested at different pH values, being practically constant at pH between 4 and 9, with a slight increase in pH 10 and a greater increase in pH 11. The removal time evaluation indicates a very fast adsorption process, removing almost 90% of BPA in the first 20 min of contact. The kinetic model indicates a monolayer formation of BPA molecules on the MSN-CTAB surface. The maximum adsorption capacity (Q max) was 155.78 mg g-1, one of the highest found in literature, and the highest for material from a renewable source.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
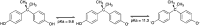



Similar articles
-
Enhanced Adsorption of Bisphenol A from Aqueous Solution with 2-Vinylpyridine Functionalized Magnetic Nanoparticles.Polymers (Basel). 2018 Oct 12;10(10):1136. doi: 10.3390/polym10101136. Polymers (Basel). 2018. PMID: 30961062 Free PMC article.
-
Rapid removal of bisphenol A on highly ordered mesoporous carbon.J Environ Sci (China). 2011;23(2):177-82. doi: 10.1016/s1001-0742(10)60391-9. J Environ Sci (China). 2011. PMID: 21516989
-
Highly Pure Silica Nanoparticles with High Adsorption Capacity Obtained from Sugarcane Waste Ash.ACS Omega. 2018 Mar 31;3(3):2618-2627. doi: 10.1021/acsomega.8b00092. Epub 2018 Mar 5. ACS Omega. 2018. PMID: 30023841 Free PMC article.
-
Adsorption performance of modified agricultural waste materials for removal of emerging micro-contaminant bisphenol A: A comprehensive review.Sci Total Environ. 2021 Aug 1;780:146629. doi: 10.1016/j.scitotenv.2021.146629. Epub 2021 Mar 20. Sci Total Environ. 2021. PMID: 34030339 Review.
-
Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents.Molecules. 2020 Aug 21;25(17):3814. doi: 10.3390/molecules25173814. Molecules. 2020. PMID: 32825791 Free PMC article. Review.
Cited by
-
Cross-Linked Chitosan/Gelatin Beads Loaded with Chlorella vulgaris Microalgae/Zinc Oxide Nanoparticles for Adsorbing Carcinogenic Bisphenol-A Pollutant from Water.ACS Omega. 2022 Jul 26;7(31):27239-27248. doi: 10.1021/acsomega.2c01985. eCollection 2022 Aug 9. ACS Omega. 2022. PMID: 35967052 Free PMC article.
-
Thermal and bisphenol-A adsorption properties of a zinc ferrite/β-cyclodextrin polymer nanocomposite.RSC Adv. 2023 Jul 20;13(32):21991-22006. doi: 10.1039/d3ra03331g. eCollection 2023 Jul 19. RSC Adv. 2023. PMID: 37483676 Free PMC article.
-
Sugarcane ash and sugarcane ash-derived silica nanoparticles alter cellular metabolism in human proximal tubular kidney cells.Environ Pollut. 2023 Sep 1;332:121951. doi: 10.1016/j.envpol.2023.121951. Epub 2023 Jun 8. Environ Pollut. 2023. PMID: 37301454 Free PMC article.
-
Adsorption of Bisphenol A onto β-Cyclodextrin-Based Nanosponges and Innovative Supercritical Green Regeneration of the Sustainable Adsorbent.Polymers (Basel). 2025 Mar 23;17(7):856. doi: 10.3390/polym17070856. Polymers (Basel). 2025. PMID: 40219247 Free PMC article.
-
The Removal of Hydrophobic Matter from Thermosensitive Poly[oligo(ethylene glycol) Monomethyl Ether Acrylate] Gel Adsorbent in Alcohol-Water Mixtures.Gels. 2022 Mar 23;8(4):200. doi: 10.3390/gels8040200. Gels. 2022. PMID: 35448101 Free PMC article.
References
-
- Dianin A. P. J. Russ. Phys.-Chem. Soc. 1891;23(523–546):601–611.
-
- Zincke T. Justus Liebigs Ann. Chem. 1905;343:75–99. doi: 10.1002/jlac.19053430106. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

