Novel drug isolated from mistletoe (1 E,4 E)-1,7-bis(4-hydroxyphenyl)hepta-1,4-dien-3-one for potential treatment of various cancers: synthesis, pharmacokinetics and pharmacodynamics
- PMID: 35516963
- PMCID: PMC9055608
- DOI: 10.1039/d0ra03674a
Novel drug isolated from mistletoe (1 E,4 E)-1,7-bis(4-hydroxyphenyl)hepta-1,4-dien-3-one for potential treatment of various cancers: synthesis, pharmacokinetics and pharmacodynamics
Abstract
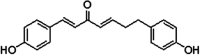
(1E,4E)-1,7-Bis(4-hydroxyphenyl)hepta-1,4-dien-3-one (DHDK) is a novel curcuminoid analogue isolated from mistletoe. DHDK exhibits better anti-tumour activity, higher bioavailability and superior stability than curcumin. DHDK is difficult to isolate from Viscum coloratum, but it can be synthesised. MTT (methylthiazolyldiphenyl tetrazolium bromide) assay was used to evaluate the in vitro cytotoxic activity of synthesised DHDK on 12 cancer cell lines. Results showed that DHDK exhibited excellent potential as an anticancer agent, especially for breast and lung cancer. Efficacy was further evaluated in vivo by using MCF-7 breast cancer models. DHDK showed a dose-dependent relationship without weight reduction, mortality growth inhibition or tissue toxicity. Pharmacokinetics and tissue distribution statistics were determined by LC-ESI-MS/MS. This work provided preliminary data on this natural compound and could open up new prospects for changing related parameters to improve drug efficacy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Network pharmacology, bioinformatics analysis, and experimental validation to reveal the target and pharmacological mechanism of DHDK in treating breast cancer.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 14. doi: 10.1007/s00210-025-04138-3. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40227311
-
Optimization of diarylpentadienones as chemotherapeutics for prostate cancer.Bioorg Med Chem. 2018 Sep 1;26(16):4751-4760. doi: 10.1016/j.bmc.2018.08.018. Epub 2018 Aug 13. Bioorg Med Chem. 2018. PMID: 30121214 Free PMC article.
-
Antiproliferative effect of an analog of curcumin bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione in human breast cancer cells.Eur Rev Med Pharmacol Sci. 2012 Dec;16(14):1900-7. Eur Rev Med Pharmacol Sci. 2012. PMID: 23242714
-
Novel diarylheptanoids of Alpinia blepharocalyx.Curr Top Med Chem. 2003;3(2):203-25. doi: 10.2174/1568026033392552. Curr Top Med Chem. 2003. PMID: 12570774 Review.
-
[Mistletoe therapy from the pharmacologic perspective].Forsch Komplementarmed. 1999 Aug;6(4):186-94. doi: 10.1159/000021248. Forsch Komplementarmed. 1999. PMID: 10529578 Review. German.
Cited by
-
Modified Curcumins as Potential Drug Candidates for Breast Cancer: An Overview.Molecules. 2022 Dec 14;27(24):8891. doi: 10.3390/molecules27248891. Molecules. 2022. PMID: 36558022 Free PMC article. Review.
-
Evaluation of the antiproliferative, cytotoxic and phytochemical properties of Zimbabwean medicinal plants used in cancer treatment.BMC Complement Med Ther. 2025 Apr 24;25(1):156. doi: 10.1186/s12906-025-04883-1. BMC Complement Med Ther. 2025. PMID: 40275320 Free PMC article.
-
Viscum coloratum (Komar.) Nakai: A Review of Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology.Biomolecules. 2025 Jul 7;15(7):974. doi: 10.3390/biom15070974. Biomolecules. 2025. PMID: 40723846 Free PMC article. Review.
-
The Anti-Inflammatory Activity of Viscum album.Plants (Basel). 2023 Mar 27;12(7):1460. doi: 10.3390/plants12071460. Plants (Basel). 2023. PMID: 37050086 Free PMC article. Review.
References
-
- World Health Organization, Cancer. Fact Sheet No. 297, http://www.who.int/mediacentre/factsheets/fs297/en/, accessed 01 August 2015
-
- Scotti L. Mendonça Júnior F. J. Ribeiro F. F. et al., Natural product inhibitors of topoisomerases: review and docking study. Curr. Protein Pept. Sci. 2018;19:275–291. - PubMed
-
- Thaloor D. Singh A. K. Sidhu G. S. et al., Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by curcumin. Cell Growth Differ. 1998;9:305–312. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

