Solvatochromism and pH effect on the emission of a triphenylimidazole-phenylacrylonitrile derivative: experimental and DFT studies
- PMID: 35517007
- PMCID: PMC9063490
- DOI: 10.1039/c9ra01275c
Solvatochromism and pH effect on the emission of a triphenylimidazole-phenylacrylonitrile derivative: experimental and DFT studies
Abstract
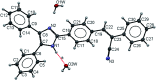
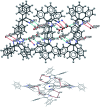
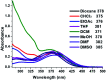
In this work, a study of the photophysical properties in different solvents and at different pH values of a luminogenic compound with a donor-π-acceptor (D-π-A) structure was carried out. The compound (Z)-3-(4-(4,5-diphenyl-1H-imidazol-2-yl)phenyl)-2-phenylacrylonitrile (2) was synthesized and characterized by SCXRD, FT-IR, 1H NMR, 13C NMR, EIMS, UV-Vis absorption and fluorescence. The SCXRD characterization reveals a monoclinic system, P21/c, with Z = 4 and an imidazole core having hydrogen bonding with respect to water molecules present in the asymmetric unit. It leads to a strong π-π-interaction in the solid state. The fluorescence λ max emission of the powder and thin film was observed at 563 nm and 540 nm respectively. Several degrees of positive solvatochromic fluorescence were observed due to different molecular conformations in various solvents. When the pH of the compound was changed with HCl or NaOH, a shift in the wavelength of emission was observed in a reversible manner. At pH 2, the λ max of emission was at 541 nm whereas at pH 14 there were two emissions at 561 nm and 671 nm. Due to their good emission in the solid state, compound 2 was tested as an emitting layer in OLEDs; the devices showed an acceptable performance with a luminance average of 450 cd m-2. The band gap was analyzed by optical absorption, cyclic voltammetry measurement and DFT calculations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures












References
-
- Sasaki S. Drummen G. P. C. Konishi G. J. Mater. Chem. C. 2016;4:2731–2743. doi: 10.1039/C5TC03933A. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

