Baseline Characteristics and Secondary Medication Adherence Patterns Among Patients Receiving Tafamidis Prescriptions: A Retrospective Analysis Using a National Specialty Pharmacy Dispensing Database
- PMID: 35517043
- PMCID: PMC9064174
- DOI: 10.2147/PPA.S352332
Baseline Characteristics and Secondary Medication Adherence Patterns Among Patients Receiving Tafamidis Prescriptions: A Retrospective Analysis Using a National Specialty Pharmacy Dispensing Database
Abstract
Introduction: Transthyretin amyloid cardiomyopathy (ATTR-CM) is a serious, underrecognized condition, which leads to heart failure and early mortality if left untreated. Until recently, heart transplantation was the only treatment for ATTR-CM. Regulatory approval of tafamidis transformed treatment for patients. In the phase 3 Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT), which established the safety and efficacy of tafamidis, medication adherence was high with 97.2% of patients taking ≥80% of scheduled doses. Evidence of real-world adherence to cardiology drugs demonstrates low adherence and suboptimal outcomes; however, real-world adherence to tafamidis has not been investigated. The main objective of this study was to describe adherence patterns of patients filling tafamidis in the Symphony Health database.
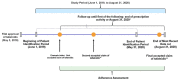
Methods: This retrospective analysis of the Symphony Health Solutions claims database used secondary adherence measures, including modified medication possession ratio (MPRm), days between fills adherence rate, and compliance rate, to assess adherence patterns of 2020 patients filling tafamidis free acid 61-mg capsules or tafamidis meglumine 4x20-mg capsules from June 1, 2019 to August 31, 2020.
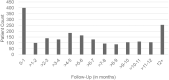
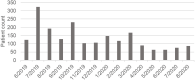
Results: Patients receiving a tafamidis formulation had characteristics consistent with the expected patient population; 71.6% were aged 75-84 years, 83.2% were male, and the highest proportion resided in the Northeast region (30.5%) of the United States. Adherence for tafamidis was high, as 75% to 100% of the patients across subgroups met or exceeded the commonly defined adherence threshold of 80%. Median number of refills ordered and received was six refills per patient. Most patients received refills with no gap (n=1633) or a gap <30 days (n=1267/1317 patients). Adherence was high across follow-up time, sex, and age subgroups. Adherence varied by geographic region, with the Northeast being significantly higher than the Midwest (mean MPRm 94.41% vs 88.21%, p=0.0007).
Conclusion: These results provide evidence that real-world adherence to tafamidis in patients with ATTR-CM is high.
Keywords: adherence; amyloidosis; cardiomyopathy; claims analysis; transthyretin amyloid.
© 2022 Roy et al.
Conflict of interest statement
Darrin Benjumea is an employee of Genesis Research who has been contracted by Pfizer, Inc. for involvement in this study. Andrew Peterson is an employee of University of the Sciences who has been contracted by Pfizer, Inc. for involvement in this study. Sapna Prasad and Alex O’Brien are employees of Clarify Health Solutions and were contracted by Pfizer, Inc. for involvement in this study. Anuja Roy, Nick Marchant, Jose Alvir, Rahul Bhambri, Jason Lynn, Yong Chen, Jason Kemner, and Bhash Parasuraman are employees of Pfizer and own stock and/or stock options. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Tafamidis medication adherence and persistence in patients with transthyretin amyloid cardiomyopathy in Japan.ESC Heart Fail. 2024 Oct;11(5):2881-2888. doi: 10.1002/ehf2.14736. Epub 2024 May 23. ESC Heart Fail. 2024. PMID: 38783561 Free PMC article.
-
Tafamidis 61 mg Patient Characteristics and Persistency? A Retrospective Analysis of German Statutory Health Insurance Data (IQVIA™ LRx).Cardiol Ther. 2024 Jun;13(2):369-378. doi: 10.1007/s40119-024-00365-6. Epub 2024 Apr 13. Cardiol Ther. 2024. PMID: 38615093 Free PMC article.
-
Baseline characteristics and secondary medication adherence among Medicare patients diagnosed with transthyretin amyloid cardiomyopathy and/or receiving tafamidis prescriptions: A retrospective analysis of a Medicare cohort.J Manag Care Spec Pharm. 2022 Jul;28(7):766-777. doi: 10.18553/jmcp.2022.28.7.766. J Manag Care Spec Pharm. 2022. PMID: 35737856 Free PMC article.
-
Tafamidis therapy in transthyretin amyloid cardiomyopathy: a narrative review from clinical trials and real-world evidence.Egypt Heart J. 2024 Jul 10;76(1):90. doi: 10.1186/s43044-024-00517-y. Egypt Heart J. 2024. PMID: 38985360 Free PMC article. Review.
-
Tafamidis: A First-in-Class Transthyretin Stabilizer for Transthyretin Amyloid Cardiomyopathy.Ann Pharmacother. 2020 May;54(5):470-477. doi: 10.1177/1060028019888489. Epub 2019 Nov 18. Ann Pharmacother. 2020. PMID: 31735059 Review.
Cited by
-
Tafamidis medication adherence and persistence in patients with transthyretin amyloid cardiomyopathy in Japan.ESC Heart Fail. 2024 Oct;11(5):2881-2888. doi: 10.1002/ehf2.14736. Epub 2024 May 23. ESC Heart Fail. 2024. PMID: 38783561 Free PMC article.
-
Tafamidis 61 mg Patient Characteristics and Persistency? A Retrospective Analysis of German Statutory Health Insurance Data (IQVIA™ LRx).Cardiol Ther. 2024 Jun;13(2):369-378. doi: 10.1007/s40119-024-00365-6. Epub 2024 Apr 13. Cardiol Ther. 2024. PMID: 38615093 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous