Microwave assisted synthesis of five membered nitrogen heterocycles
- PMID: 35517065
- PMCID: PMC9057010
- DOI: 10.1039/d0ra05150k
Microwave assisted synthesis of five membered nitrogen heterocycles
Abstract
Our continuously changing environment demands sensible and sustainable chemistry. Consequently, organic synthesis started to follow green chemistry principles in recent years. It is observed that microwave (MW) radiation has been widely used as a source of energy in organic synthesis in the past decade. The MW heating approach has evolved into a new green method in organic synthesis since it provides short reaction time, high yields, and high product purities along with a decrease in the rate of by-product formation. Solvent-free reaction protocols worked well under MW irradiation. All these features make MW assisted organic synthesis an environment-friendly approach. In organic synthesis, heterocycles are vital targets especially nitrogen-containing ones because of their prominent presence in natural products and widespread applications in pharmaceutical industries. Five membered nitrogen heterocycles include pyrroles, oxazoles, pyrrolidones, etc. among which pyrroles are the most important ones due to their potent biological properties. Even though there are a variety of reaction protocols for the synthesis of pyrroles, a significant development materialized in MW assisted synthesis of pyrroles in the past few years. In this review, we focus on the developments in MW assisted synthesis of pyrroles and other five-membered nitrogen heterocycles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

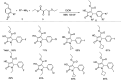
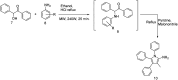
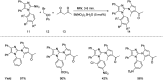



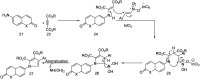

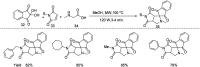
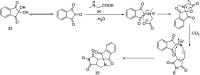
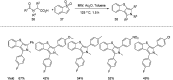
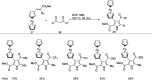
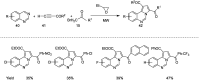

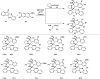
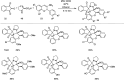
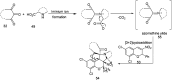



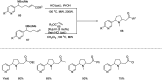
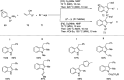
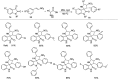
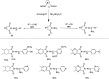

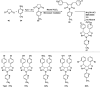
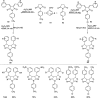




References
-
- Bhardwaj V. Gumber D. Abbot V. Dhiman S. Sharma P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015;5:15233–15266. doi: 10.1039/C4RA15710A. - DOI
-
- La Regina G. Coluccia A. Bai R. Famiglini V. Pelliccia S. Passacantilli S. Mazzoccoli C. Ruggieri V. Sisinni L. Bolognesi A. Maria Rensen W. Miele A. Nalli M. Alfonsi R. Di Marcotullio L. Gulino A. Brancale A. Novellino E. Dondio G. Vultaggio S. Varasi M. Mercurio C. Hamel E. Lavia P. Silvestri R. New pyrrole derivatives with potent tubulin polymerization inhibiting activity as anticancer agents including hedgehog-dependent cancer. J. Med. Chem. 2014;57:6531–6552. doi: 10.1021/jm500561a. - DOI - PMC - PubMed
-
- Battilocchio C. Poce G. Alfonso S. Cesare Porretta G. Consalvi S. Sautebin L. Pace S. Rossi A. Ghelardini C. Di Cesare Mannelli L. Schenone S. Giordani A. Di Francesco L. Patrignani P. Bava M. A class of pyrrole derivatives endowed with analgesic/anti-inflammatory activity. Bioorg. Med. Chem. 2013;21:3695–3701. doi: 10.1016/j.bmc.2013.04.031. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

