Gold(iii) promoted formation of dihydroquinazolinones: double X-H activation by gold
- PMID: 35517079
- PMCID: PMC9056963
- DOI: 10.1039/d0ra06537d
Gold(iii) promoted formation of dihydroquinazolinones: double X-H activation by gold
Abstract
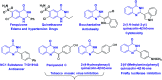
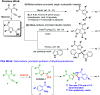
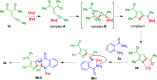
An efficient 2-furyl gold-carbene promoted synthetic method was developed for the formation of dihydroquinazolinones from enynones by dual insertion of anthranilamides. In this organic transformation a new C-O and two C-N bond formations occurred and dihydroquinazolinones were obtained with a quaternary centre in moderate to very good yields in one-pot synthesis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Gold-carbene assisted formation of tetraarylmethane derivatives: double X-H activation by gold.Org Biomol Chem. 2019 May 15;17(19):4856-4864. doi: 10.1039/c9ob00470j. Org Biomol Chem. 2019. PMID: 31038501
-
Silver(I)-Catalyzed Tandem Reaction of Enynones and 4-Alkenyl Isoxazoles: Synthesis of 2-(Furan-2-yl)-1,2-dihydropyridines.J Org Chem. 2023 Jun 2;88(11):7038-7045. doi: 10.1021/acs.joc.3c00312. Epub 2023 May 15. J Org Chem. 2023. PMID: 37183921
-
Stereoselective Synthesis of Tetrasubstituted Furylalkenes via Gold-Catalyzed Cross-Coupling of Enynones with Diazo Compounds.Org Lett. 2017 Jul 7;19(13):3482-3485. doi: 10.1021/acs.orglett.7b01467. Epub 2017 Jun 13. Org Lett. 2017. PMID: 28609108
-
Palladium-Catalyzed Oxidative Cross-Coupling of Conjugated Enynones with Allylarenes: Synthesis of Furyl-Substituted 1,3-Dienes.J Org Chem. 2019 Jun 21;84(12):8275-8283. doi: 10.1021/acs.joc.9b00922. Epub 2019 Jun 4. J Org Chem. 2019. PMID: 31117574
-
Synthetic strategy with representation on mechanistic pathway for the therapeutic applications of dihydroquinazolinones.Eur J Med Chem. 2016 Nov 10;123:596-630. doi: 10.1016/j.ejmech.2016.08.001. Epub 2016 Aug 3. Eur J Med Chem. 2016. PMID: 27517807 Review.
References
-
- Brown E. G., Ring Nitrogen and Key Biomolecules, The Biochemistry of N-heterocycles, Kluwer Academic, Boston, 1998
- Majumdar K. C. Karunakar G. V. Sinha B. Synthesis. 2012;44:2475. doi: 10.1055/s-0032-1316566. - DOI
- Ye Z. Shi L. Shao X. Xu X. Xu Z. Li Z. J. Agric. Food Chem. 2013;61:312. doi: 10.1021/jf3044132. - DOI - PubMed
-
- Mühlbauer V. Dallmeier D. Brefka S. Bollig C. Voigt-Radloff S. Denkinger M. Dtsch. Arztebl. Int. 2019;116:23. - PMC - PubMed
- Kamble A. A. Kamble R. R. Chougala L. S. Kadadevarmath J. S. Maidur S. R. Patil P. S. Kumbar M. N. Marganakop S. B. ChemistrySelect. 2017;2:6882. doi: 10.1002/slct.201700498. - DOI
-
- Shang X.-F. Morris-Natschke S. L. Liu Y.-Q. Guo X. Xu X.-S. Goto M. Li J.-C. Yang G.-Z. Lee K.-H. Med. Res. Rev. 2018;38:775. doi: 10.1002/med.21466. - DOI - PMC - PubMed
- Luth M. R. Gupta P. Ottilie S. Winzeler E. A. ACS Infect. Dis. 2018;4:301. doi: 10.1021/acsinfecdis.7b00276. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

