Potent in vivo antimalarial activity of water-soluble artemisinin nano-preparations
- PMID: 35517081
- PMCID: PMC9057047
- DOI: 10.1039/d0ra05597b
Potent in vivo antimalarial activity of water-soluble artemisinin nano-preparations
Abstract
Artemisinin is a remarkable compound whose derivatives and combinations with multiple drugs have been utilized at the forefront of malaria treatment. However, the inherent issues of the parent compound such as poor bioavailability, short serum half-life, and high first-pass metabolism partially limit further applications of this drug. In this study, we enhanced the aqueous phase solubility of artemisinin by encapsulating it in two nanocarriers based on the polymer polycaprolactone (ART-PCL) and lipid-based Large Unilamellar Vesicles (ART-LIPO) respectively. Both nanoformulations exhibit in vitro parasite killing activity against Plasmodium falciparum with the ART-LIPO performing at comparable efficacy to the control drug solubilized in ethanol. These water-soluble formulations showed potent in vivo antimalarial activity as well in the mouse model of malaria at equivalent doses of the parent drug. Additionally, the artemisinin-PCL nanoformulation used in combination with either pyrimethamine or chloroquine increased the survival of the Plasmodium berghei infected mice for more than 34 days and effectively cured the mice of the infection. We highlight the potential for polymer and liposome-based nanocarriers in improving not only the aqueous phase solubility of artemisinin but also concomitantly retaining its therapeutic efficacy in vivo as well.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
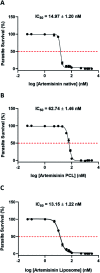


References
-
- WHO, World Malaria Report 2019, https://apps.who.int/iris/rest/bitstreams/1262394/retrieve, Page No. 10 and 11, accessed May 17, 2020
-
- Fu W. Chinese Medicine and Culture. 2018;1:18–20.
LinkOut - more resources
Full Text Sources

