Novel edaravone-based azo dyes: efficient synthesis, characterization, antibacterial activity, DFT calculations and comprehensive investigation of the solvent effect on the absorption spectra
- PMID: 35517118
- PMCID: PMC9056902
- DOI: 10.1039/d0ra06934e
Novel edaravone-based azo dyes: efficient synthesis, characterization, antibacterial activity, DFT calculations and comprehensive investigation of the solvent effect on the absorption spectra
Abstract
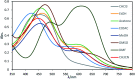
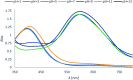
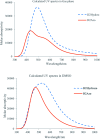
The present study deals with designing and synthesizing novel dyes using the drug combination of edaravone and azo compounds which can be used as an indicator for anions and cations. The desired product synthesis was accomplished via a two-step process involving diazotizing the aromatic amines followed by the resultant salts coupling with edaravone. The resulting dyes were obtained with high yields under mild conditions. The structures of the dyes were identified with UV-vis, FT-IR, 1H NMR and 13C NMR spectra and CHN analysis. To investigate the solvatochromism effect, the interaction of different solvents with the selected dyes was evaluated using several parameters including the dielectric constant, refractive index, hydrogen bond donating ability, hydrogen bond accepting ability and dipolarity/polarizability scale. To achieve deep understanding about the stability and geometrical characteristics of the azo-hydrazo tautomers of the synthesized dyes and their UV-visible spectra prediction, some DFT calculations were also carried out on the synthesized dyes. The antibacterial activities of some synthesized compounds were also evaluated using the disk diffusion method. The results revealed different activity of the selected synthesized dyes for antibacterial tests against selected Gram positive and Gram negative bacteria.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Synthesis and evaluation of changes induced by solvent and substituent in electronic absorption spectra of some azo disperse dyes.Spectrochim Acta A Mol Biomol Spectrosc. 2012 Apr;89:238-42. doi: 10.1016/j.saa.2011.12.062. Epub 2011 Dec 29. Spectrochim Acta A Mol Biomol Spectrosc. 2012. PMID: 22261112
-
Synthesis of Some New Heterocyclic Azo Dyes Derived from 2-amino-3-cyano-4.6- diarylpyridines and Investigation of its Absorption Spectra and Stability using the DFT.Curr Org Synth. 2021;18(5):506-516. doi: 10.2174/1570179417666201218164519. Curr Org Synth. 2021. PMID: 33342417
-
Experimental and Computational Study of Novel Pyrazole Azo Dyes as Colored Materials for Light Color Paints.Materials (Basel). 2022 Aug 11;15(16):5507. doi: 10.3390/ma15165507. Materials (Basel). 2022. PMID: 36013644 Free PMC article.
-
Synthesis, structural characterization and tautomeric properties of some novel bis-azo dyes derived from 5-arylidene-2,4-thiazolidinone.Spectrochim Acta A Mol Biomol Spectrosc. 2014 May 21;126:105-11. doi: 10.1016/j.saa.2014.02.010. Epub 2014 Feb 15. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24594883
-
Unveiling the structural aspects of novel azo-dyes with promising anti-virulence activity against MRSA: a deep dive into the spectroscopy via integrated experimental and computational approaches.RSC Adv. 2025 Jan 20;15(3):1665-1679. doi: 10.1039/d4ra06367h. eCollection 2025 Jan 16. RSC Adv. 2025. PMID: 39835211 Free PMC article.
Cited by
-
Synthesis, Identification, and Biological Study for Some Complexes of Azo Dye Having Theophylline.ScientificWorldJournal. 2021 Jul 21;2021:9943763. doi: 10.1155/2021/9943763. eCollection 2021. ScientificWorldJournal. 2021. PMID: 34335115 Free PMC article.
References
-
- Abrahart E., Dyes and their Intermediates, Edward Arnold, London, 1971
-
- Hubner K. Chem. Unserer Zeit. 2006;40:274–275. doi: 10.1002/ciuz.200690054. - DOI
-
- Hunger K., Mischke P., Rieper W., Raue R., Kunde K. and Engelet A., “Azo Dyes” in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. 2005
-
- Soares G. M. B. Amorim M. T. P. Hrdina R. Costa-Ferreira M. Process Biochem. 2002;37:581–587. doi: 10.1016/S0032-9592(01)00244-8. - DOI
-
- Chen C. J. Liu G. Y. Liu X. S. Li D. D. Ji J. New J. Chem. 2012;36:694–701. doi: 10.1039/C2NJ20882B. - DOI
LinkOut - more resources
Full Text Sources

