Construction of magnetic MgFe2O4/CdS/MoS2 ternary nanocomposite supported on NaY zeolite and highly efficient sonocatalytic degradation of organic pollutants
- PMID: 35517154
- PMCID: PMC9058412
- DOI: 10.1039/d0ra08831e
Construction of magnetic MgFe2O4/CdS/MoS2 ternary nanocomposite supported on NaY zeolite and highly efficient sonocatalytic degradation of organic pollutants
Abstract
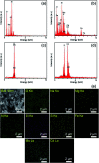
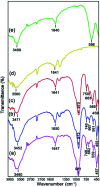
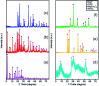
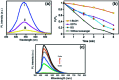
In this work, the novel magnetically separable NaY zeolite/MgFe2O4/CdS nanorods/MoS2 nanoflowers nanocomposite was successfully synthesized through the ultrasonic-assisted solvothermal approach. FESEM, EDAX, XRD, FTIR, TEM, AFM, VSM, N2-BET, UV-vis DRS and PL were utilized to identify the as-synthesized nanocomposite. Subsequently, the sonocatalytic activity of this nanocomposite was assessed in the degradation of organic dyes, including methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) from water solutions for the first time. Several analytical parameters like irradiation time, process type, initial MB concentration, H2O2 concentration, catalyst dosage, organic dye type, and US power have been systematically investigated to attain the maximum sonocatalytic yield. Regarding the acquired data, the NaY/MgFe2O4/CdS NRs/MoS2 NFs sonocatalyst was incredibly able to completely eliminate the MB via engaging the US/H2O2 system. The kinetic evaluates demonstrated the sonodegradation reactions of the MB followed a first-order model. The apparent rate constant (k app) and half-life time (t 1/2) acquired for the sonodegradation process of MB utilizing the US/H2O2/NaY/MgFe2O4/CdS NRs/MoS2 NFs system were measured to be 1.162 min and 0.596 min-1, respectively. The free ˙OH radicals were also recognized as the main reactive oxygen species in the MB sonodegradation process under US irradiation. In addition, the outcomes of the recyclability study of the NaY/MgFe2O4/CdS NRs/MoS2 NFs sonocatalytic clearly displayed a less than 6% drop of the catalytic activity in up to four sequential runs. Lastly, a plausible mechanism for the sonodegradation reaction of organic dyes was suggested and discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










References
LinkOut - more resources
Full Text Sources
Miscellaneous

