Unravelling the true MOF-5 luminescence
- PMID: 35517225
- PMCID: PMC9053756
- DOI: 10.1039/d0ra02509g
Unravelling the true MOF-5 luminescence
Abstract
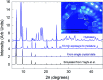
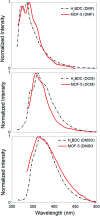
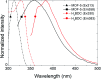
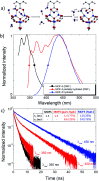
Highly pure millimeter-sized MOF-5 single crystals were synthesized and characterized. Photoluminescence (PL) spectroscopy and time-correlated single photon counting (TCSPC) demonstrate a solvent-guest dependency of MOF-5 emission and its ligand-centred nature. These results allow measuring the true MOF-5 luminescence free of solvent at a wavelength of 355 nm, a significantly lower wavelength than previously published. MOF-5 emission was also evaluated with different solvents and various degrees of water intake, explaining previously published observations. Comparison between lifetimes shows the fluorophore stabilization within the frameworks and demonstrates the progressive influence of the Zn4O subunits on the fluorescence during hydration. Overall, this work highlights the necessity to obtain phase-pure material, especially when moisture sensitivity can play a role, before ascribing electronic transitions. This study is a rigorous new take on the iconic MOF-5 and on its photoluminescence properties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Lin R. B. Liu S. Y. Ye J. W. Li X. Y. Zhang J. P. Adv. Sci. 2016;3:1–20. - PubMed
LinkOut - more resources
Full Text Sources

