Dispersion-induced structural preference in the ultrafast dynamics of diphenyl ether
- PMID: 35517230
- PMCID: PMC9053750
- DOI: 10.1039/d0ra02224a
Dispersion-induced structural preference in the ultrafast dynamics of diphenyl ether
Abstract
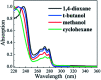
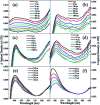
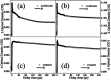
Dispersion interactions are omnipresent in large aromatic systems and influence the dynamics as intermolecular forces. The structural preference induced by dispersion interactions is demonstrated to influence the excited state dynamics of diphenyl ether (DPE) using femtosecond time-resolved transient absorption (TA) associated with quantum chemical calculations. The experimental results in aprotic solvents show that the S1 state is populated upon irradiation at 267 nm with excess vibrational energy dissipating to solvent molecules in several picoseconds, and then decays via internal conversion (IC) within 50 ps as well as intersystem crossing (ISC) and fluorescence with a lifetime of nanoseconds. The polarity of the solvent disturbs the excited state energies and enhances the energy barriers of the ISC channel. Furthermore, the intermolecular dispersion interactions with protic solvents result in the OH-π isomer dominating in methanol and the OH-O isomer is slightly preferred in t-butanol in the ground state. The hydrogen bonded isomer measurements show an additional change from OH-O to OH-π geometry in the first 1 ps besides the relaxation processes in aprotic solvents. The time constants measured in the TA spectra suggest that the OH-O isomer facilitates IC. The results show that the OH-π isomer has a more rigid structure and a higher barrier for IC, making it harder to reach the geometric conical intersection through conformer rearrangement. This work enables us to have a good knowledge of how the structural preference induced by dispersion interactions affects excited state dynamics of the heteroaromatic compounds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Solvent-polarity dependence of ultrafast excited-state dynamics of trans-4-nitrostilbene.Phys Chem Chem Phys. 2024 Jan 3;26(2):788-807. doi: 10.1039/d3cp05245a. Phys Chem Chem Phys. 2024. PMID: 38088777
-
Effect of hydrogen bonding on the nonradiative properties of dibenzofuran.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Jan 5;224:117466. doi: 10.1016/j.saa.2019.117466. Epub 2019 Aug 10. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 31422340
-
Multi-spectroscopic and theoretical analyses on the diphenyl ether-tert-butyl alcohol complex in the electronic ground and electronically excited state.Phys Chem Chem Phys. 2017 Jul 21;19(27):18076-18088. doi: 10.1039/c7cp02967e. Epub 2017 Jul 4. Phys Chem Chem Phys. 2017. PMID: 28675201
-
Excited-state dynamics of nitroperylene in solution: solvent and excitation wavelength dependence.J Phys Chem A. 2008 May 1;112(17):3823-30. doi: 10.1021/jp800254q. Epub 2008 Mar 27. J Phys Chem A. 2008. PMID: 18366202
-
Ultrafast excited-state dynamics of copper(I) complexes.Acc Chem Res. 2015 Mar 17;48(3):782-91. doi: 10.1021/ar500353h. Epub 2015 Feb 3. Acc Chem Res. 2015. PMID: 25646861 Review.
Cited by
-
Unraveling the effect of solvents on the excited state dynamics of C540A by experimental and theoretical study.RSC Adv. 2023 Feb 8;13(8):4924-4931. doi: 10.1039/d3ra00259d. eCollection 2023 Feb 6. RSC Adv. 2023. PMID: 36762085 Free PMC article.
References
-
- Lehn J. M. Angew. Chem., Int. Ed. 1988;27:89–112. doi: 10.1002/anie.198800891. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

