Reactive intermediates in copanlisib metabolism identified by LC-MS/MS: phase I metabolic profiling
- PMID: 35517257
- PMCID: PMC9060959
- DOI: 10.1039/c8ra10322d
Reactive intermediates in copanlisib metabolism identified by LC-MS/MS: phase I metabolic profiling
Abstract
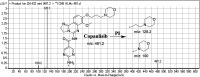
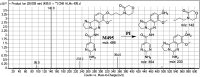
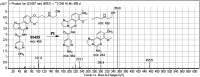
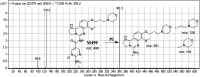
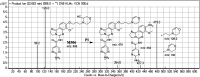
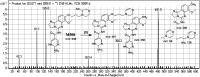
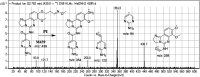
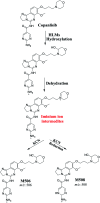
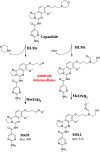
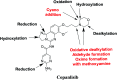
Copanlisib (CNB; Aliqopa™) is a novel, intravenous phosphoinositide 3-kinase inhibitor used to treat various solid and hematological malignancies. CNB was recently approved by the U.S. FDA to treat adults that relapsed after two preceding systemic therapies. Using LC-MS/MS, we screened for the in vitro metabolites of CNB formed in human liver microsomes (HLMs) and probed for the generation of reactive electrophiles using methoxyamine and potassium cyanide as nucleophiles to capture reactive electrophiles by forming stable adducts that are suitable for identification by LC-MS/MS. Seven CNB phase I metabolites generated by oxidation, hydroxylation, oxidative dealkylation, reduction, and N-oxidation were identified. In addition, four reactive electrophiles, 2 aldehydes and 2 iminium ions, were identified, and a prediction of the corresponding bioactivation mechanism is presented. The formation of reactive metabolites may be associated with the side effects reported for CNB. To our knowledge, this is the first report on the detailed structural characterization of reactive intermediates generated in CNB metabolism.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Reactive intermediates in naquotinib metabolism identified by liquid chromatography-tandem mass spectrometry: phase I metabolic profiling.RSC Adv. 2019 Apr 1;9(18):10211-10225. doi: 10.1039/c9ra00224c. eCollection 2019 Mar 28. RSC Adv. 2019. PMID: 35520926 Free PMC article.
-
Investigation of Fenebrutinib Metabolism and Bioactivation Using MS3 Methodology in Ion Trap LC/MS.Molecules. 2023 May 22;28(10):4225. doi: 10.3390/molecules28104225. Molecules. 2023. PMID: 37241965 Free PMC article.
-
LC-ESI-MS/MS reveals the formation of reactive intermediates in brigatinib metabolism: elucidation of bioactivation pathways.RSC Adv. 2018 Jan 3;8(3):1182-1190. doi: 10.1039/c7ra10533a. eCollection 2018 Jan 2. RSC Adv. 2018. PMID: 35540908 Free PMC article.
-
Role of cyclic tertiary amine bioactivation to reactive iminium species: structure toxicity relationship.Curr Drug Metab. 2011 Jan;12(1):35-50. doi: 10.2174/138920011794520044. Curr Drug Metab. 2011. PMID: 21222587 Review.
-
Copanlisib: First Global Approval.Drugs. 2017 Dec;77(18):2057-2062. doi: 10.1007/s40265-017-0838-6. Drugs. 2017. PMID: 29127587 Review.
Cited by
-
IOA-244 is a Non-ATP-competitive, Highly Selective, Tolerable PI3K Delta Inhibitor That Targets Solid Tumors and Breaks Immune Tolerance.Cancer Res Commun. 2023 Apr 14;3(4):576-591. doi: 10.1158/2767-9764.CRC-22-0477. eCollection 2023 Apr. Cancer Res Commun. 2023. PMID: 37066023 Free PMC article.
-
Characterization of Stable and Reactive Metabolites of the Anticancer Drug, Ensartinib, in Human Liver Microsomes Using LC-MS/MS: An in silico and Practical Bioactivation Approach.Drug Des Devel Ther. 2020 Nov 30;14:5259-5273. doi: 10.2147/DDDT.S274018. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33299299 Free PMC article.
-
Identification of Iminium Intermediates Generation in the Metabolism of Tepotinib Using LC-MS/MS: In Silico and Practical Approaches to Bioactivation Pathway Elucidation.Molecules. 2020 Oct 28;25(21):5004. doi: 10.3390/molecules25215004. Molecules. 2020. PMID: 33126762 Free PMC article.
References
-
- Morschhauser F. Bron D. Bouabdallah K. Vitolo U. Linton K. Van Den Neste E. Mappa S. Giurescu M. Childs B. H. Zinzani P. L. Blood. 2013;122:87.
LinkOut - more resources
Full Text Sources

