Cubosomes with surface cross-linked chitosan exhibit sustained release and bioavailability enhancement for vinpocetine
- PMID: 35517286
- PMCID: PMC9060951
- DOI: 10.1039/c8ra10302j
Cubosomes with surface cross-linked chitosan exhibit sustained release and bioavailability enhancement for vinpocetine
Abstract
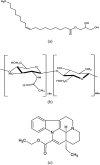
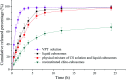
The present study aims to develop cubosomes with surface cross-linked chitosan for sustained drug delivery and enhanced oral bioavailability of vinpocetine (VPT). GMO based liquid cubosomes with VPT loading were prepared by the high pressure homogenization method. In order to enhance the anti-digestion effect, chitosan was cross-linked on cubosomes by the Schiff reaction, followed by solidification via spray drying. The obtained spray-dried cubosomes (chito-cubosomes) are spherical microspheres with nano-sized holes on the surface. After reconstitution, the particle size and zeta potential of chito-cubosomes were determined to be ∼250 nm and +35.9 mV, respectively. In comparison to unmodified liquid cubosomes, chito-cubosomes exhibited a significant anti-digestion effect with a typical sustained release profile. In comparison to a VPT suspension, liquid cubosomes showed a 2.5-fold higher C max and 3.0-fold higher AUC0-∞, while chito-cubosomes further enhanced bioavailability (5.0-fold) with prolonged MRT (2.2-fold) and delayed T max (2.8-fold). The results suggested that chito-cubosomes could be a promising drug carrier for enhancing oral absorption with sustained release behavior.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
LinkOut - more resources
Full Text Sources

