Facile one-pot synthesis of heterostructure SnO2/ZnO photocatalyst for enhanced photocatalytic degradation of organic dye
- PMID: 35517351
- PMCID: PMC9054812
- DOI: 10.1039/d0ra03233f
Facile one-pot synthesis of heterostructure SnO2/ZnO photocatalyst for enhanced photocatalytic degradation of organic dye
Abstract
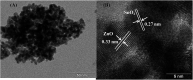
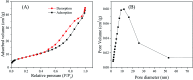
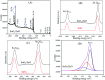
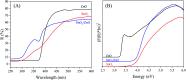
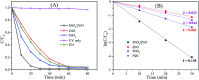
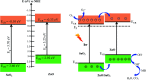
In this work, heterostructure SnO2/ZnO nanocomposite photocatalyst was prepared by a straightforward one step polyol method. The resulting photocatalysts were characterized by X-ray diffraction (XRD), nitrogen adsorption-desorption analyses, transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and UV-vis diffuse reflectance spectroscopy (UV-vis DRS). The results showed that the synthesized SnO2/ZnO nanocomposites possessed mesoporous wurtzite ZnO and cassiterite SnO2 nanocrystallites. The photocatalytic activity of the prepared SnO2/ZnO photocatalyst was investigated by the degradation of methylene blue dye under UV light irradiation. The heterostructure SnO2/ZnO photocatalyst showed much higher photocatalytic activities for the degradation of methylene blue dye than individual SnO2, ZnO nanomaterials and reference commercial TiO2 P25. This higher photocatalytic degradation activity was due to enhanced charge separation and subsequently the suppression of charge recombination in the SnO2/ZnO photocatalyst resulting from band offsets between SnO2 and ZnO. Finally, these heterostructure SnO2/ZnO nanocatalysts were stable and could be recycled several times without any appreciable change in degradation rate constant which opens new avenues toward potential industrial applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
On behalf of all authors, the corresponding author declares that there is no conflict of interest.
Figures




References
-
- Al-Kdasi A. Idris A. Saed K. Guan C. T. Global NEST J. 2004;6:222–230. doi: 10.30955/gnj.000288. - DOI
LinkOut - more resources
Full Text Sources

